Sternum Plating System
A small percentage of surgical procedures that involve splitting the sternum to enter the thoracic cavity. develop serious complications that require sternal repair and/or reconstruction.
The new Sternal Fixation System is intended as a solution to encourage bony union in these cases. It serves as a solution for surgeons looking to rigidly fixate these patients with the added safety of emergent re-entry.
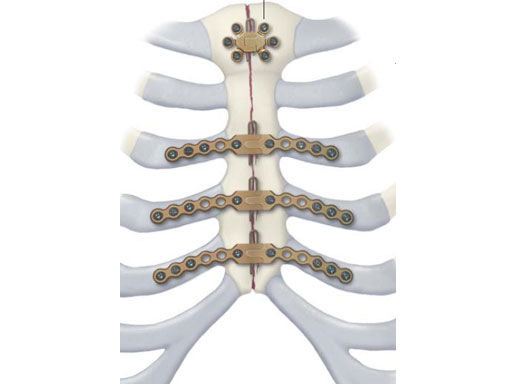
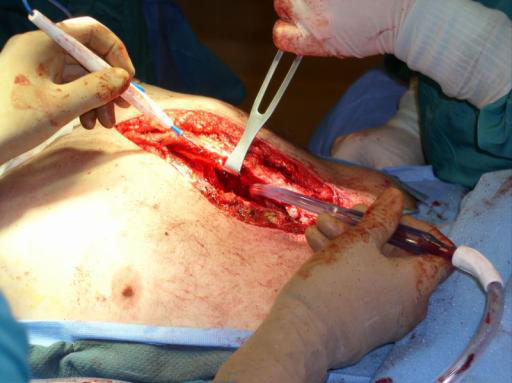
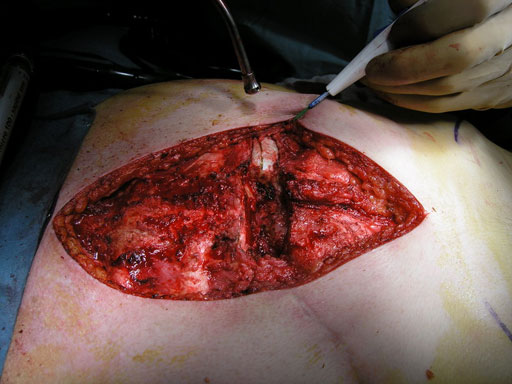
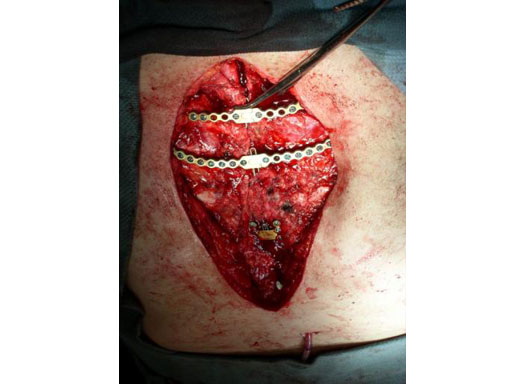
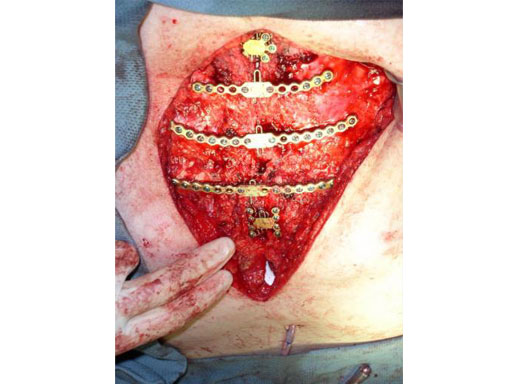
While many surgeons currently remove the sternum the new system allows for a procedure that involves reducing the sternal halves and implanting plates, which span the sternum and are fixed to the ribs and/or sternum of the patient. The chest wall is then reinforced through advancement of the pectoral muscles.
The Sternal Fixation System consists of titanium locking plates that function with 3.0 mm titanium locking screws, similar to those used in the mandible area. The straight plates are actually an assembly of two single plates, which are connected by a U shaped pin. In case of emergency removing the pin allows re-entry into the thoracic cavity. Long and straight plates are fixed to the ribs; additional smaller plates are available for fixation of the manubrium.
Implants
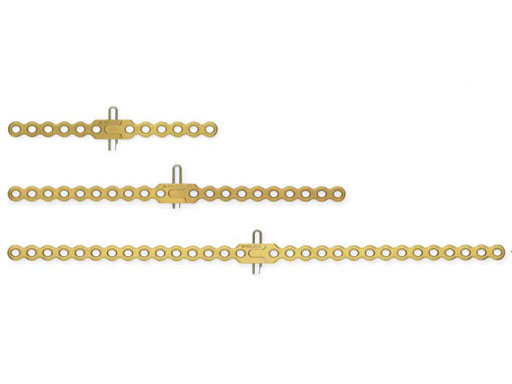
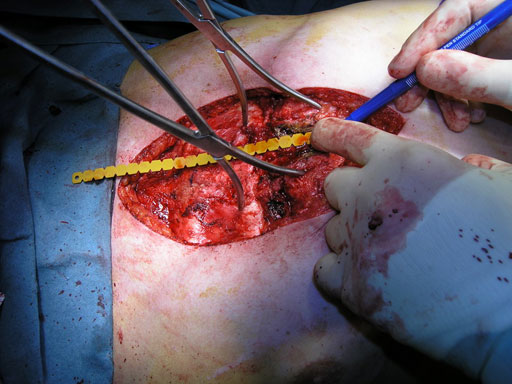
The Sternal Fixation System is available with 12-hole Titanium Locking Plates (Fig 2) as well as Titanium Locking H plates small/large and Titanium Locking Star Plates with 6 and 12 holes (Fig 3). The set contains Locking Screws (3.0 mm) from 8 mm to 18 mm and the Titanium Emergency Release Pin to connect and disconnect the plates at the fixation site,
A Titanium Sternal Locking Plate with 30 holes is available additionally (Fig 2).
Instruments
The Sternal Fixation System Set contains a 2.4/3.0 mm Screwdriver with Holding Sleeve, a combination Bending Pliers for 2.0 mm/2.4 mm plates, a Bending Template with 37 holes as well as a Plate Cutter. For effective reduction of the sternotomy there are special Sternal Reduction Forceps and additional large Bone Reduction Forceps available.
The set also comes with respective threaded drill guides and drill bits with stops from 8 mm to 18 mm (J-Latch).
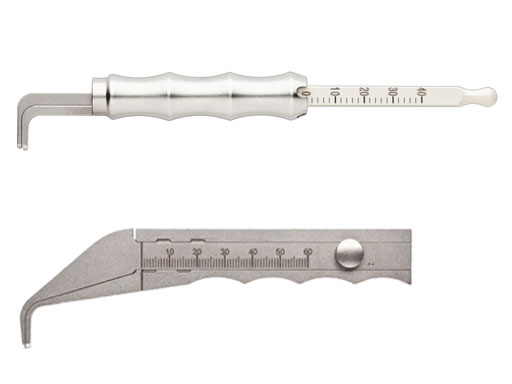
Caliper
The new universal caliper is designed for easy readability, durability, and compact size. In comparison with the currently available flat caliper this device also offers improved handling capabilities yet is easier to clean. The calibrated markings are etched in black on an off-white arm extension, which has a square shape and is etched on all four sides. The extensions arm is made of radel (polyphenylsulfone (PPSU) for better durability. It is intended for various areas of thoracic surgery, ie, with the sternal fixation system and the matrix rib system.
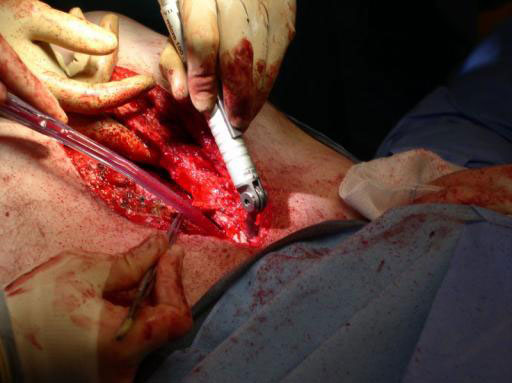
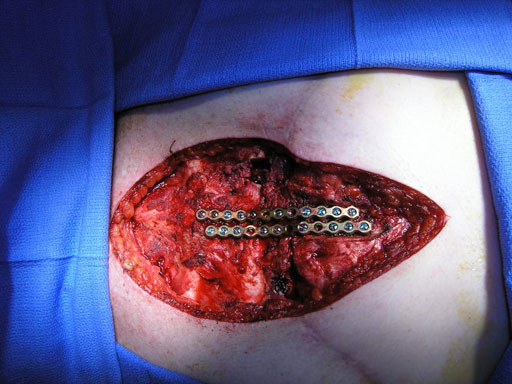
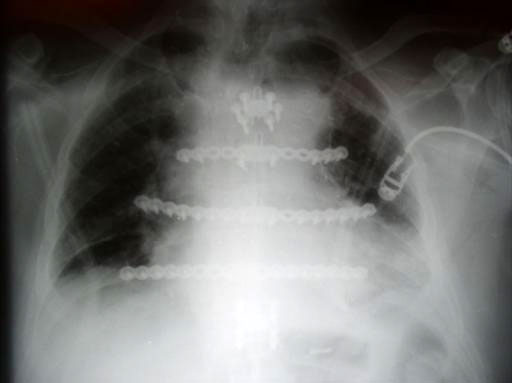
Sternal closure with Ti Plate system
Cases provided by J Thornton, UT Southwestern Medical Center, Dallas, TX
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.