Mandible External Fixator II
After the release of the first mandible external fixator in July 2004 the feedback from clinicians, oftentimes requesting more technical ease and versatility, has lead to the development of the mandible external fixator II.
Although the mandible external fixator II system comes with many new features, it addresses the same clinical indications as the original system:
- Severe open mandibular fractures
- Highly comminuted closed fractures
- Nonunions and delayed unions (especially associated with infection)
- Tumor resections
- Facial deformity correction
- Gunshot wounds
- Panfacial fractures
- Burn maintenance
- Bone grafting defects
A strong impetus from surgeons was the request for an increased number of snap-on clamps in the kit. Thus instead of originally 8 clamps the new system contains 12 of the newly developed parallel clamps. Distinguishable by their purple color half these clamps can be used with two 2.0mm K-wires to secure small fragments, e.g. in the condylar process. For this purpose 2.0 K-wire are now included in the set as well.
For the percutaneous instrumentation a new drill guide & obturator for 2.0 mm & 2.5 mm K-wires has been developed.
The new cannula & orbturator is 1 cm longer than its predecessor. The set contains self-drilling anatomic Schanz screws with two thread lengths and a shoulder stop to prevent overinsertion. The shaft length of the screws is adapted to the local skin thickness and with respect to the regional geometry of the mandible (symphysis, body, ramus). The shaft of the Schanz screws allow for using devices with quick coupling.
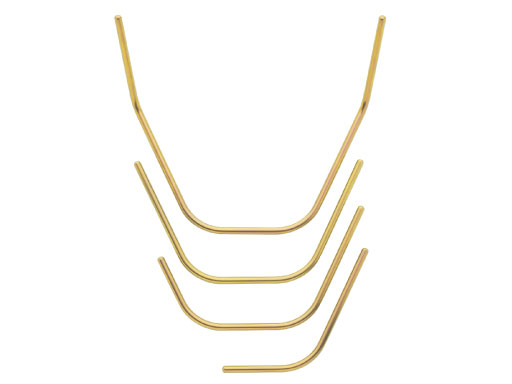
The 4.0 mm titanium connecting rods are available in four sizes (full mandible, full mandible with ramus, three-quarter mandible, and one-half mandible). Anatomically pre-bent to the mandibular shape the metal rods cover a wide anatomic variety but can be contoured to match individual patient needs with the included rod bender.
To ensure an optimal stability of the framework construction the connecting rods should be positioned approximately one fingerbreadth away from the patients skin surface, evenly around the mandibular circumference, in order to keep the cantilevers along the Schanz screws short. At least two Schanz screws should be placed on large segments: One in close proximity (10 mm) to the fracture or resection line and another one preferably another 10 mm away from it.
To allow the surgeon to build a longer modular frame, a new 120 mm carbon fiber connectingrod has been included in the set. Extended carbon fiber rods with lengths of 140, 160, 180, and 200 mm are available on request, but will not fit inside the tray of the graphics case.
New Instruments specifically designed for this system are the ratcheting Screwdriver Handle, the rapid driver and the Schanz screw adaptor for tightening of the Schanz screws.
The new mandible external fixator II can be adjusted throughout the whole OR procedure and is MR safe.
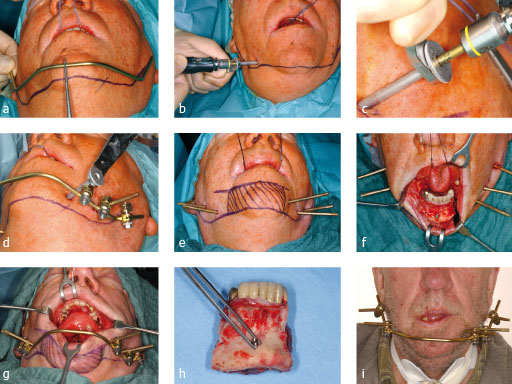
Fig 1ai: Stabilization of the mandible during primary resection of a floor-of-the-mouth carcinoma with infiltration of the sympyseal bone. The sequence shows the application starting in the angles, stepwise assembly, temporary removal of the connecting bar for en bloc tumor resection and remounting.
Case images courtesy of Carl-Peter Cornelius, Mnchen, DE
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.