VetFix System (Clamp-Rod Internal Fixator)
State of the art treatment of long bone fractures in animals mandatesas in human medicinethat a wide variety of implants are available around the clock. Therefore, a versatile system, requiring only a minimum of instruments and implants, has been desperately sought. In the late nineties, the AO Development Institute in Davos in conjunction with the Expert Group of AO VET (VEEG), the veterinary section of the AO Foundation, initiated the development of the VetFix System. The following requirements for the new system were set by the VEEG: simple in its design, user friendly, costs within an acceptable range, and available in different sizes. The VetFix system consists of a round, roughened stainless steel rod with a longitudinal laseredged line. The rod accepts special symmetrical clamps that slide onto it to any desired position. The clamps can be rotated to either side of the rod to accept cortex screws of the desired length (Fig 1). The VetFix System, manufactured from the same implant quality stainless steel as used for the Dynamic Compression Plates, was developed in the following four sizes (named after the cortex screws that they accept): 4.5/5.5, 3.5, 2.7, and 2.0 mm (Fig 2). End clamps were developed to prevent the bar moving within the clamps (Fig 3). At the moment, only the 3.5, 2.7, and 2.0 mm systems exist for small animals.
Discussion
The VetFix provides a free arrangement of clamps that enables the surgeon to choose the screw position according to the fracture configuration and the bony contours. A recent comparison of two 4.5/5.5 mm VetFix systems using six and ten clamps respectively on a rod of the length of the broad 10 -hole DCP revealed no significant differences in stiffness and strength. This range allows the surgeon to concentrate on the fracture situation and choose the clamp setup that is the most appropriate for the situation.
The clamps were tested in a separate mechanical test setup comparing smooth and roughened (sand blasted) rod surfaces under dry and moistened conditions. Their holding power was tested in rod rotation and pull out and compared to each other. On the basis of these results, the best combinations were chosen for serial production. The VetFix System may be contoured to the surface of the bone in all directions at the same time.
The VetFix System allows compression between fragments only by using lag screw technique. Direct axial compression of the fracture cannot be achieved with the VetFix System. It is used as a neutralization fixation. The VetFix is similar to a so-called biological fracture fixation system, which represents state of the art fracture treatment in humans and is also widely accepted in small animal trauma surgery. The VetFix is a low contact internal fixation system that is thought to maintain superior blood supply to the periosteum underneath the implant.
Using a rod in connection with the clamps makes the VetFix a more voluminous implant than any of the corresponding DCP implants. The diameter reaches its maximum over the clamps, which may increase soft tissue irritation. Preliminary observations in this respect based on fascia and skin closure, showed no strenuous skin tension when the humerus, radius, femur, or tibia were treated. In the metacarpal/metatarsal area, skin closure may lead to moderate tension.
The AO Veterinary Expert Group decided on the name VetFix: Clamp-Rod Internal Fixator (CRIF).
Drill Guide
For proper centering of the screws in the screw holes of the clamps, the Universal Drill Guides are preferably used (Fig 1). They have a rounded tip on one side with a centered hole to accept the corresponding drill. This tip fits into the screw holes of each clamp. Inside this centering guide is an inner gliding drill guide which, equipped with a spring mechanism, makes the contact with the bony cortex and guides the drill in the correct direction. On the other side of the drill guide handle, the corresponding tap sleeve can be found. This Universal Drill Guide is available for all system sizes.
Holding Forceps
The Holding Forceps contain a ratchet mechanism based on a knurled screw to grasp and secure the rod for contouring, applying the clamps over the rod and for manipulating the implant within the wound.
Clamp Forceps
These forceps were developed to facilitate applying the Standard Clamps to the implant rod. These forceps are opened, inserted with their blunt tip into the screw hole of the clamp and released, which presses the tip into the slit between the two clamp flanges and in doing so, opens the clamp, widens the clamp ring, and allows frictionless sliding of the clamp over the rod. It can be used during assembling of the implant and later for repositioning clamps within the surgical field.
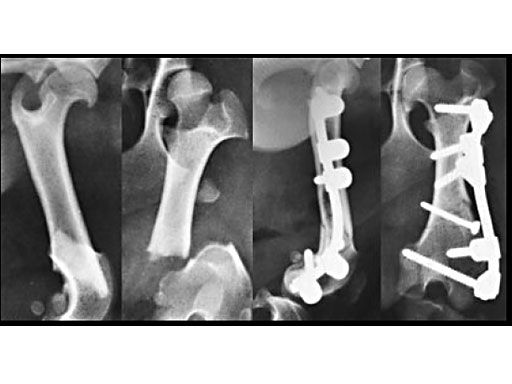
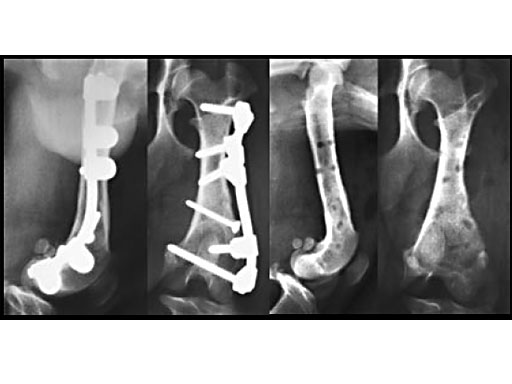
Clinical application in small animals: A 1-year-old female Dachshund was admitted with a transverse fracture in the distal metaphysis of the femur. Fixation was accomplished with a 2 mm interfragmentary screw in a lag fashion and a 2.7 mm VetFix with five screws. The fracture healed and the implants were removed four months postoperatively. The benefit of the VetFix can be shown on this breed of dog, the females of which do not have one straight bone in their body. The rod was contoured along the bone, which is not possible with a plate.
Anvil for Bending Pliers
The anvil for bending pliers for the clamp rod internal fixator (CRIF) assists with contouring of the 3.0 mm and 5.0 mm rods. Acute bends of 1015 mm radii can be created. The rod is held securely, preventing slippage during bending. Deformation marks on the rod are minimized. The anvil is made out of hardened stainless steel and works with the existing bending pliers.
1-year-old Dachshund
(Case provided by K. Zahn and U. Matis, Munich, Germany)
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.