PHILOS
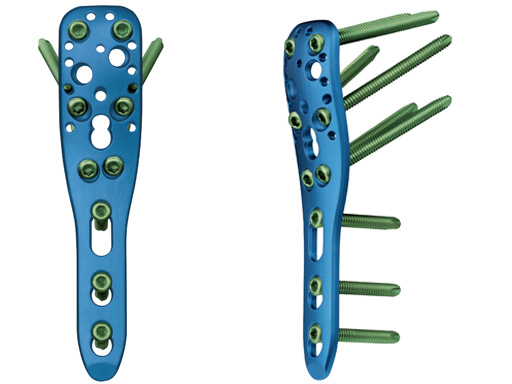
The new angular stable device (available as on request only) is a low profile precontoured plate for the proximal humerus (proximal part of the plate is a little longer in comparison to LPHP) and is indicated like the LPHP for subcaptial two- and threepart fractures, but can also be used for fourpart fractures. Due to its low profile the plate can be applied very proximally. At the proximal part of the plate there are additional small holes for the fixation of sutures which can be applied into the rotator cuff tendons to neutralize muscle forces. In comparison to the LPHP the PHILOS has additional oblique screws for the humeral head.
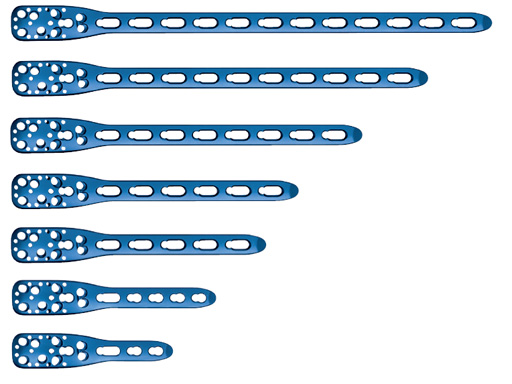
PHILOS is indicated for the treatment of all kinds of proximal humeral fractures, especially in poor bone. Now, a longer and stronger plate, PHILOS long, has been designed to treat proximal humeral fractures with extension into the shaft, fractures after new trauma just below an osteosynthesis, and nonunions.
The proximal part of the PHILOS long is identical to the standard plate. This ensures 100% compatibility of all existing PHILOS instruments. It also ensures that the surgeon can use exactly the same surgical technique for the proximal part as for the standard PHILOS. The entire shaft of PHILOS long is equipped with long holes. This allows maximal flexibility for both the lag screws for the reduction of the fracture and the locking head screws for angular stable fixation. It is recommended to use locking head screws in the shaft to reach maximum stability.
PHILOS long is reinforced significantly over the standard PHILOS and the LCP T-Plate. Increase in strength is about 50%. Plate undercuts improve vascularization of the periost by reducing contact between implant and bone. A bullet tip has been added to allow easier application of the minimally invasive surgical technique.
PHILOS long is available in titanium and stainless steel.
The PHILOS sizing templates are a simple solution for the intraoperative selection of implant size. They are designed for surgeons working with sterile packaged implants where a visual comparison of the implant to the fracture pattern is impossible without opening the package and to ensure maximum precision in determining the correct implant size.
The templates are available in three lengths, equivalent to PHILOS 3-holes, 5-holes, and long. The outer contours reflect the shape and size of the implants. They can be washed and sterilized. Temporary positioning with 1.6?mm K-wire is possible.
2.8 mm LCP Drill Guide Long and 2.8 mm Calibrated Drill Bit The 2.8 mm LCP Drill Guide Long, and the 2.8 mm Calibrated Drill Bit are used together for easy drilling and measuring of 3.5 mm locking head screws in the 3.5 mm LCP plates and the proximal humerus plates (PHILOS). The length can be measured directly from the drill guide and drill bit to save time during the OR.
The 2.8 mm LCP Drill Guide is 118 mm long which enables the surgeon to grip it more easily. The 2.8 mm Calibrated Drill Bit is 248 mm long. The drill bit features a 2.8 mm drill tip and a 4.5 mm body with calibration.
The 4.5 mm body of the drill bit allows ease of reading the calibration.
The calibration measures from 1095 mm in a bold etch. The design of the drill guide and drill bit ensures that the measurement is accurate, and therefore, the screw will be in the appropriate length.
Additionally, the new proximal suture holes on the PHILOS plate are penetrating the plate from the top to the sides, allowing the attachment of the suture after the plate has been fully mounted to the bone.
3.5 mm LCP Percutaneous Aiming System for PHILOS
PHILOS is usually implanted through the deltopectoral approach which implicates a long incision and extensive soft-tissue retraction to reach the anatomy of interest. The less-invasive transdeltoid approach is more soft-tissue friendly and gives a better view of the greater tubercle.
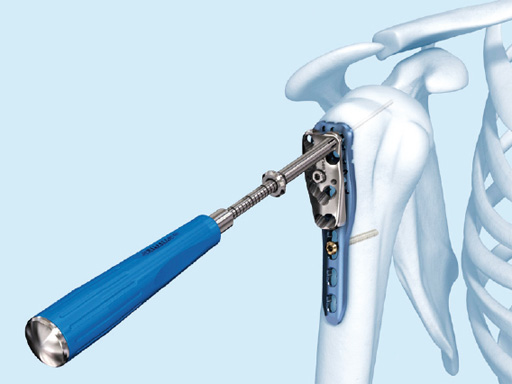
The 3.5 mm LCP percutaneous aiming system for PHILOS offers the possibility to insert the PHILOS plate through the transdeltoid approach and to insert the shaft screws percutaneously enabling a less-invasive application of PHILOS.
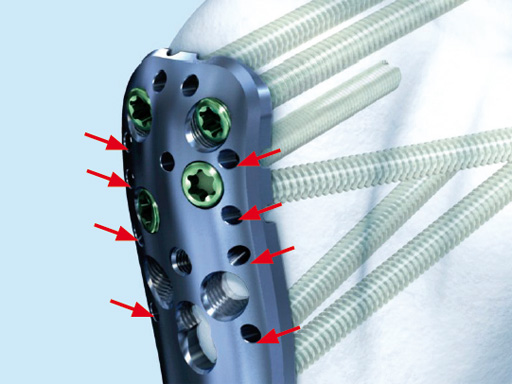
The system consists of a sleeve system and an aiming arm. It is used analogously to the existing PHILOS aiming instruments and other aiming systems. The aiming arm is radiolucent to allow control under image intensifier. Locking as well as cortex screws can be used through the device. Compression achieved by cortex screws in the shaft may lead to plate tension. The device has to be locked to the plate at both ends to ensure the platedevice alignment.
A safe zone is defined to protect the axillary nerve (screw holes near the axillary nerve are not accessible through the device). Therefore, the elongated plate hole is not accessible through the aiming arm due to the protected nerve zone. However, by abduction of the arm after fixation, these screw holes become accessible.
Overall, the 3.5 mm LCP percutaneous aiming system facilitates plate insertion as well as positioning and adjustment.
PHILOS Instrument Upgrade
One of the main complications in proximal humeral plating is primary screw perforation. Primary screw perforation happens if too long screws are set at the time of surgery, despite intraoperative image intensifier control. Using measuring notations on drill bits and K-wires seems inadequate for achieving reliable screw length in osteopenic bone. Therefore, dedicated instruments were developed to drill the lateral cortex only, followed by the use of a depth probe to feel the resistance of the subchondral bone in these patients.
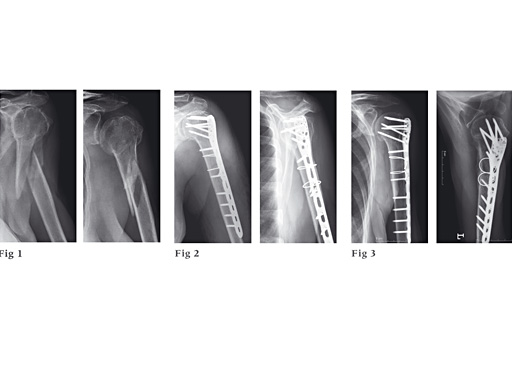
87-year-old woman fell down the stairs, fracture type 12-C1.
Fig 1
Preoperative x-ray.
Fig 2
Postoperative x-ray.
Fig 3
Follow-up after 6 months.
With kind permission of Michael Plecko, MD.
A 76-year-old woman suffered a low-energy fall at home.
Fig 1 Preoperative x-ray.
Fig 3 Preliminary fixation and percutaneous reduction with bone elevator.
Fig 4 Intraoperative view.
Fig 5 Intraoperative view of final result.
Fig 6 Postoperative view. Note the minimally invasive approach.
Fig 7 Postoperative result.
Case provided by Stefaan Nijs, Leuven, Belgium
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.