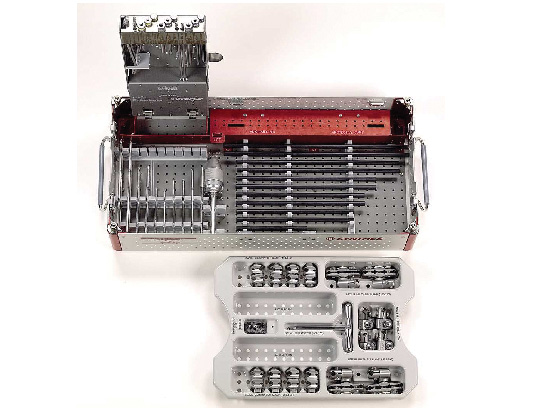
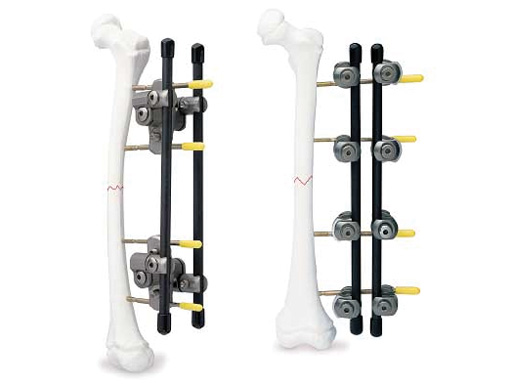
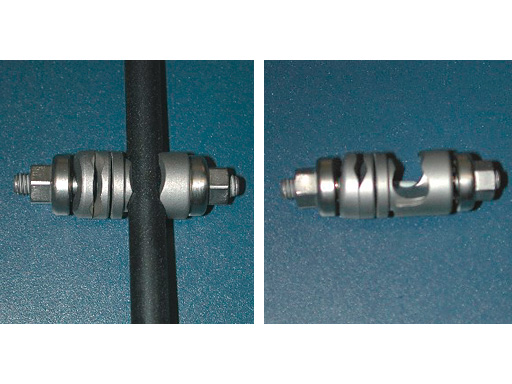
Medium External Fixator
The Medium External Fixator is part of the AO ExFix system. The complete concept consists of three lines with different dimensions each:
- Tubular/ Rod Modular System
- Unibody System / MEFiSTO
- Hinged/ Adjustable Fixators
Compatibility of all lines and dimensions is ensured. All clamps are available as open and snap-on version. The indications are very flexible depending on the experience of the surgeon and the material available.
The indications for the Medium ExFix are
- Forearm and wrist with new clamps and/or the 8mm Distal Radius Fixator
- Humerus, distal tibia and ankle joint of small adults and children
The operation technique is similar to the 11mm system. The 3 tube modular technique is strongly recommended as standard method.
Rods and Rod Attachments
The Modular Tube/Rod External Fixator of the AO is now available in four sizes: Large (11mm), Medium (8mm), Small (4mm), and Mini (2mm).
The Large, Medium and Small Ex Fix have similar clamps, rods and screws:
- Single Pin Clamps for pin to rod/tube connection
- Multi Pin Clamps for multiple pins
- Combination Clamps:
- for rod/tube to rod/tube connection
- for pin to rod/tube connection
- for pin to pin connection (in future)
Tube and rods are available in steel and/or carbon fiber. The Schanz screws are self-tapping or self-drilling in steel and titanium. All clamps will be open and snap on. The clamps will be colour coded for each size. The standard frame is the 3 Tube Modular Technique.
Lately, the Medium External Fixator was completed. The Medium Ex Fix is indicated for the stabilization of medium sized bones: the foot, forearm, elbow, humerus and small adults tibia and childrens long bones. The combination up to the Large 11mm and down to the Small 4mm System is possible and one of the main indications. Indications for the Medium Ex Fix are identical to the classic indications for external fixation: fractures with severe soft tissue damage (closed and/or open), polytrauma, septic cases etc.
Rods and Rod Attachments
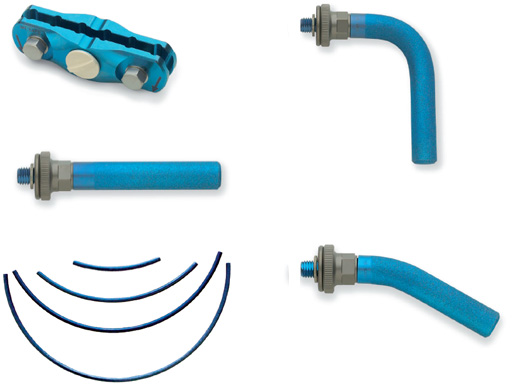
The Medium and Large External Fixator family now offer a wider variety of possible constructs to create the appropriate frame configuration suitable to the fracture and soft-tissue situation. The line extension features new Pin Clamps, Outrigger Posts, and Curved Carbon Fiber Rods.
This enables the creation of bilateral frames where the rods are spaced further apart, allowing more stable constructs. The wider attachment points on the clamps allow sufficient space around the soft tissues. The number of tightening points to control the position of the rod is reduced from two to only one when using the Outrigger Posts and Pin Clamps. This reduction can be secured more quickly and easily. The frame construct can be double-stacked or made into a delta frame while using only one Pin Clamp in each fragment.
4- and 6-Position Pin Clamps are now available in both medium (8 mm) and large (11 mm) size. All four Pin Clamps use the same subcomponents as the vise plate assemblies from the corresponding Multi-Pin Clamps. In place of the Rod Attachments, the threaded holes of the Pin Clamps are left empty to be filled with Outrigger Posts. The Pin Clamps connect two or more Schanz screws in one fragment of the bone and provide attachment sites for up to two Outrigger Posts. The Outrigger Posts are available in a straight, 30 bend and 90 bend in large and medium size. The design allows for any orientation of the Outrigger Post in a complete 360 arc. The Outrigger Posts and the Pin Clamps are MR Safe and compatible with the existing External Fixation lines.
The 8 mm and 11 mm diameter Curved Carbon Fiber Rods come with a 45, 90, 135, and 180 bend. They may be used in pelvic fractures and whenever extra clearance is needed due to swelling or damage of the soft tissues.
Clamps
MR safe external fixation clamps and carbon fibre rods allow patients to undergo MRI without the need to remove clamps and rods, maintaining the fracture reduction. Therefore, AO decided to upgrade all External Fixator Systems to become MR safe by using 3/6 L implant stainless steel and Ti-6A1-4V material. With the new clamps, clips, and rod attachments, the Medium External Fixator is now available as an MR safe system. The range of applications has been broadened to treat pediatric and small stature adults. The improved snap-on fit offers three tightening options: a knurled nut, an internal and an external hex. The Large and Small External Fixators as well as the Distal Radius Fixator are already MR safe.
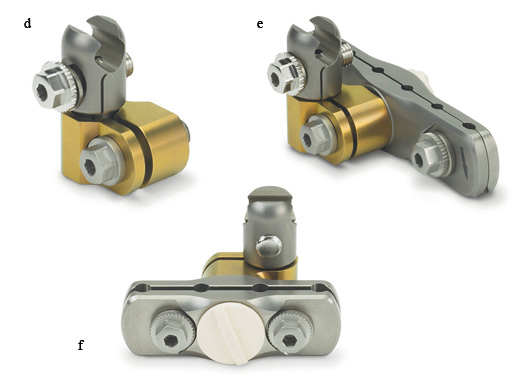
a) Medium Combination Clamp, MR safe.
b) 8.0 mm/11.0 mm Combination Clamp, MR safe.
c) Medium Multi-Pin Clamp, 4-position, MR safe.
d) Rod Attachment for Medium Multi-Pin Clamp, MR safe.
e) Medium Multi-Pin Clamp, 6-position, MR safe.
f) Medium Multi-Pin Clamp, 4-position, MR safe.
Medium Open Compressor
The Medium Open Compressor is an intraoperative and postoperative instrument which clamps onto the rods of an external fixation frame and provides compression or distraction. It is similar in design to the existing Large Open Compressor and Small Open Compressor which are used for the Large (11 mm diameter) and Small (4 mm) External Fixators. The Medium Open Compressor operates with either an 8 mm or 6 mm diameter rod making it compatible with the Medium External Fixator and the Low Profile Wrist Fixator. The Medium Open Compressor comes in stainless steel. One turn achieves 1 mm of either compression or distraction. It has an 8 mm external hex and an 3.5 mm internal hex.
Small Universal Chuck with T-Handle
The Small Universal Chuck with T-Handle is used to insert Schanz screws or wires from 1.0 - 6.0 mm diameters. The size of the T-Handle has been reduced for lighter weight and easier handling and will be available for all External Fixation Systems.
Combination T-Wrench, 8 mm
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.