Materials used for AO implants - An overview and outlook
Introduction
AO implants are manufactured from a wide range of different materials. This article will provide you with an overview of the most commonly used materials and explain their clinical advantages.
The majority of metallic AO trauma implants are manufactured from Cr-Ni-Mo stainless steel, CP titanium, or Ti-6Al-7Nb alloy. A few cobalt-base alloys with commercial names such as L-605 and Elgiloy are also used for specialty implants. Nonmetallic implant materials include PEEK (polyetheretherketone) and resorbable polylactide polymers. Influences on specific material selection during the implant design phase include anatomical location, perceived stress limits, diagnostic imaging considerations, competitive factors, and most importantly, the capability to solve a clinical problem. Calcium sulfate, calcium phosphate, and other bioceramics used for bone grafting or as bone void fillers will not be covered in this review article.
Stainless steel
Implant quality 316L stainless steel meeting International Organization for Standardization (ISO), American Society for Testing and Materials (ASTM) and AO ASIF compositional, metallurgical, and mechanical requirements is used for a large number of fracture fixation devices. The wide combination of mechanical properties is ideal for a variety of implants. Some of the product features include:
- Cerclage wireability to twist and deform without breaking.
- Reconstruction plates3-D contourability.
- DHSgood fatigue strength.
- Bone screwsexcellent torsional strength and ductility.
- Bone plateshigh strength with good ductility.
The positive attributes of implant quality 316L (ISO 5832-1) are offset by a few deficiencies including the possibility of nickel allergy due to the 15% nickel content and considerable signal artifact during MRI that may interfere with diagnostic imaging. The use of Fast Spin Echo pulse sequence during MRI can reduce the amount of artifact obtained with stainless steel. Low-nickel implant stainless compositions that contain a maximum 0.05% nickel are emerging to address the nickel sensitivity problem. AOCID recently coordinated a literature survey at the Technical University Munich on low nickel sensitization in animals and humans and an AO Research Grant is funding a paravertebral patch test study of Ni-sensitized patients in Germany. Fortunately, the low-nickel implant alloys exhibit improved mechanical properties and corrosion resistance. Typical annealed mechanical properties are as follows:
| Low Nickel | ISO 5832-1 | |
| UTS (MPa) | 1000 | 590 |
| 0.2% YS (MPa) | 600 | 250 |
| Elong (%) | 50 | 57 |
| ROA (%) | 70 | 88 |
| Fatigue (MPa) | 480 | 180 |
UTS = Ultimate Tensile Strength
YS = Yield Strength
Elong = Elongation
ROA = Reduction Of Area
Titanium
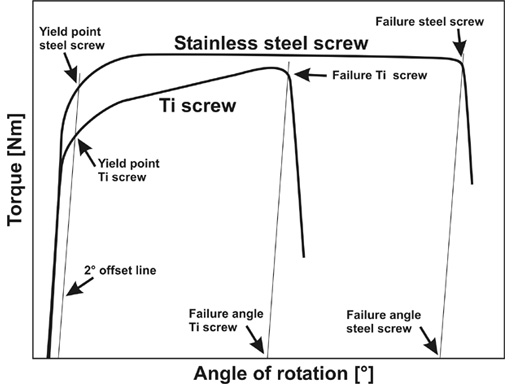
Pure titanium is considered the benchmark by which all other biomaterials are judged due to its outstanding combination of long term corrosion resistance and biocompatibility. The low amount of MR artifact and ability to be anodized for color-coded implant systems are unique properties of titanium. Pure titanium can be cold worked for added strength but the majority of trauma applications include relatively low-stressed maxillofacial, cranial, and hand implants. Its overall mechanical properties are somewhat inferior to stainless steel. A schematic of torque versus angle of rotation for titanium and stainless steel bone screws highlights the lower torsional yield strength and ductility associated with pure titanium.
Fig
Torque versus angle of rotation for stainless steel and titanium bone screws.
Titanium alloys
a+ titanium alloys such as Ti-6Al-7Nb offer increased strength for highly stressed AO implants such as cannulated and solid IM nails, universal spine clamps, LISS plates, thoracolumbar rods, and cannulated screws. They offer improved strength but less tensile and bending ductility when compared to pure titanium. Ti-15Mo is a relatively new titanium alloy with moderate strength, high ductility, and excellent notch sensitivity.
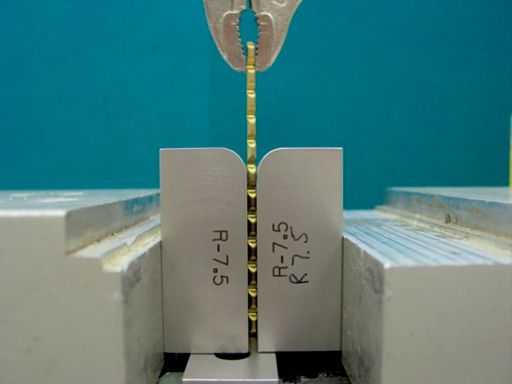
Mechanical property studies have been performed with various Ti Grade 4 and Ti-15Mo plates in order to compare the reverse bending properties. Reverse bend testing (n=3) was performed with a series of annealed Ti Grade 4 and beta annealed Ti-15Mo plates according to ISO 7801.
| Mean number of bends | ||
| Ti Grade 4 | Ti-15Mo | |
| Locking reconstruction | 11 3 | 32 5 |
| Universal fracture | 4 0 | 10 2 |
| 2.0 Locking | 19 3 | 56 4 |
The reverse bend fracture resistance of the Ti-15Mo plates was improved by a factor of 2.5-3X when compared to Ti Grade 4 plates. Enhanced reverse bending performance represents an important clinical advantage during intra-operative plate contouring.
Fig
Reverse bend testing.
PEEK
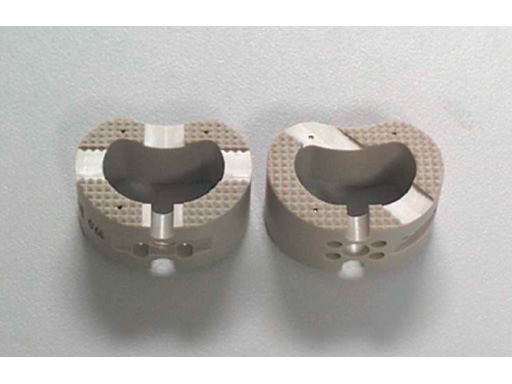
PEEK is an advanced thermoplastic polymer that is available as an implantable material. Special synthesis methods and processing precautions control the composition, uniformity, and internal cleanliness. Current applications include vertebral spacers, spiked washers, and other implants are under development. PEEK offers good mechanical properties (100 MPa YS; 20% elongation; 170 MPa flexural strength) and radiolucency.
PEEK spiked washers are formulated with 6% barium sulfate for radiopacity as a replacement for the polyoxymethylene (POM) C spiked washer with stainless steel reinforcement ring. Unconventional machining and cleaning procedures are required to provide noncontaminated surfaces during fabrication operations. PEEK mechanical properties will not be degraded during steam autoclaving, ETO, or gamma sterilization.
Fig
PEEK vertebral spacers.
Resorbable polylactide
The generalized chemical formula for polylactide polymer is (C3H4O2)n. Various isomers known as L-lactide, D-lactide, and DL-lactide refer to the structural orientation of the polymer. Isomers can be differentiated on the basis of their specific optical rotation. Amorphous (noncrystalline) 70:30 L/DLpolylactide is the primary stereoisomer used for mid-face and cranial resorbable plates, screws, and burr hole covers. The in vivo degradation mechanism is well-documented in the literature.
PLA -> lactic acid -> water + CO2
Handling operations are critical since polylactide granules are supplied in inert gas purged foil packs, stored at a low temperature, vacuum dried at a high temperature, and transferred under inert gas cover to injection molding or compression molding equipment.
Resorbable polymers are ideal for craniofacial implants because of their small mass and the low applied stress. Their excellent vascularity and 46 week fracture healing timeframe are favorable clinical factors. Material improvements such as higher strength and faster resorption rate plus improved implant designs will offer expanded opportunities in the future for resorbable polymers.
Surface modification
Implant surface interactions are primarily responsible for biological response and have a pronounced effect on the clinical performance of trauma products. Ongoing research by the AO Research Institute has identified the importance of metallic implant surface microtopography on the cellular reactions that are obtained. Movement between implant surface and soft tissue may cause fibrous capsule formation around a liquid filled void on stainless steel. The liquid phase allows buildup of cellular detritus, fretting debris, and possible infection. Capsule formation is not observed on titanium implants. Recent findings in Davos indicate that there is a strong correlation between lack of fine microroughness and the presence of a liquid filled void. Results have supported the hypothesis that stainless steel void formation is due to lack of microtopography and the inability of cells to adhere to surface discontinuities. Other surface modifications for implants include low friction anodizing to improve the fretting and galling resistance of titanium. Bulk coatings include HA to encourage biological fixation and antiseptic or antibiotic antibacterial films. Osteoinductive additives such as BMP-2, IGF-1, and TGF-1 will be applied to implant surfaces in the future to control specific biological functions.
Future developments
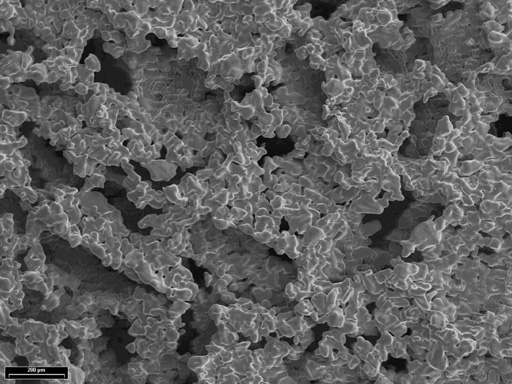
Substantial efforts have been made by many research groups to develop metallic foams that provide low stiffness, stable bony ingrowth, structural support, and delivery of bone forming compounds. Long-range developments are also under investigation to explore advanced material technologies such as:
- Nonmagnetic amorphous metals with high strength and good wear properties.
- Titanium shape memory alloys that do not contain nickel.
- Nanotechnology processing to produce CP titanium with strength levels that exceed Ti-6Al-7Nb.
- Novel titanium alloys that demonstrate an ultralow elastic modulus, extremely high strength, and super plasticity due to a dislocation-free plastic deformation mechanism.
Potential clinical applications for these new materials include implants with low apparent density for osteoporotic bone, improved MR or CT imaging, in vivo shape memory activation, and better resistance to fatigue fracture.
Fig
Example of titanium foam structure.
Conclusion
Successful integration of implant materials for AO implant applications is the result of close cooperation between clinicians, research scientists, material specialists, product development designers, and manufacturing engineers. Clinical feedback especially through the medical AO Expert Groups is crucial to understand the advantages, disadvantages, and limitations of conventional and advanced biomaterials. Active participation within the ISO and ASTM implant committees ensures that high quality AO material standards will be maintained on a worldwide basis. Full manufacturing support by the producers is needed to determine processing response and cost-effective manufacturing strategies for new implant materials. This team effort within the AO is responsible for providing surgical implants with improved properties and superior clinical performance.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.