Humerus Block
Humerusblock study
The Humerusblock was made available to a selection of surgeons in November 2000 as part of a documentation series. The aim of this documentation series was to record the results of first applications with the new implant.
In the context of this series 16 cases were documented. The fractures included 7 subcapital humerus fractures, 6 subcapital humerus fractures with additional fracture of the greater tubercle and three 4-fragment fractures. A closed surgical procedure was performed in all cases. The surgeons generally made use of K-wires with a diameter of 2.2 mm and no thread. The use of additional screws was necessary in 8 cases.
Follow-up assessment was carried out in 14 of the 16 cases. In 13 cases fracture consolidation was described by the treating physician; in one case delayed healing was suspected. Slight impaction was observed in four cases and more severe impaction in two cases. Impaction can be regarded as a desirable event. In relation to the implant, the K-wires act as a sort of splint so that impaction occurs in a controlled manner. Dislocation of the humeral head was not observed in any case. In 11 patients, the K-wires perforated the articular surface; this occurred 6 times intraoperatively and 5 times postoperatively. Primary and secondary perforation of the K-wires through the articular surface indicates that the K-wires need to be placed in the immediate subchondral region in porotic bone if they are to find sufficient anchorage. Therefore, implant removal after fracture healing or prior to mobilization is necessary; the surgeon may possibly have to withdraw some of the K-wires slightly early on. The following complications occurred during the healing process: local wound irritation above the implant (2 cases; due to incorrectly clipped K-wires) and skin perforation by a K-wire (1 case). In one case, reduction of the greater tubercle was lost in a 4-fragment fracture; reoperation to stabilize the fragment was necessary. There was probably delayed union in one case of a 76-year-old patient. Infections and / or nerve lesions were not observed in any case.
In contrast to conventional K-wire osteosynthesis, the Humerusblock permitted the surgeons to perform adequate stable fixation of proximal humerus fractures. Compared with fracture treatment by plate osteosynthesis, this procedure is far less invasive with maximal conservation of the fracture fragments residual vascularity. The conclusions that can be drawn from this series are limited by the small number of patients included (n=16) and followed up (n=14). It can be stated that it proved of value in all the participating centers and that with further application a set of suitable indications will no doubt emerge.
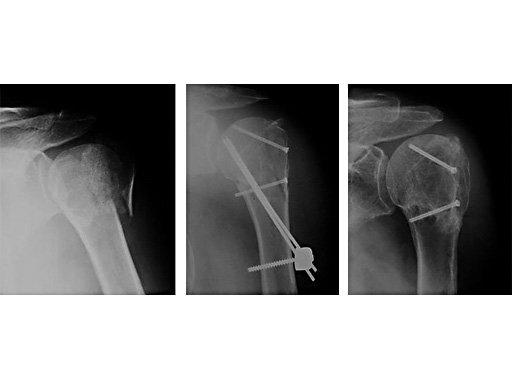
62 year old female patient
Fig 1
Accident
Fig 2
postoperative
Fig 3
Fracture healed
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.