SynMesh
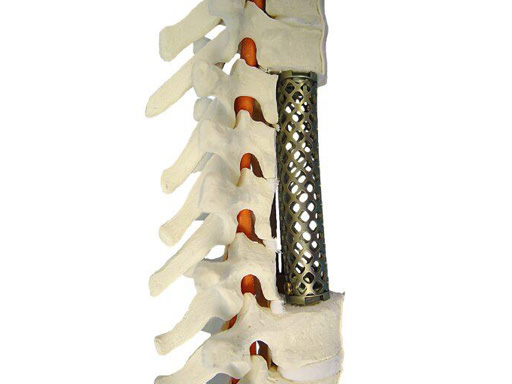
The SynMesh system is indicated for use as a vertebral body replacement device in thoracolumbar spine (T1 through L5) to replace a collapsed, damaged, or unstable vertebral body due to tumor or trauma. This modular system allows building of the most appropriate construct to address the defect and patients anatomy. The SynMesh system is to be used with Synthes supplemental internal fixation systems, eg, VentroFix or USS.
This update features 36 additional mesh heights and additional instruments.
SynMesh: additional sizes (noncontoured and precontoured)
SynMesh is used for vertebral body replacement in the case of tumor or trauma from T1L5. Existing sizes are cut to fit in corpectomy sites, which increases OR time. Straight implants in longer vertebral body reconstructions can impinge posteriorly on the spinal cord because they do not follow the normal anatomy. Therefore, additional heights have been developed to reduce the need to cut down larger sizes and reduce OR time in these cases. The 10 mm diameter SynMesh is now available in heights of 14, 16, 20, 22, 24, and 32 mm, the 12 mm diameter in heights of 14, 16, 20, 22, and 24 mm, and the 15 mm diameter in heights of 18, 20, 22, and 24 mm. Contoured implants are offered to better fit the anatomy and reduce the likelihood of impingement on the spinal cord. They are available in diameters 12 mm, 15 mm, 17 x 22 mm, 22 x 28 mm, and 26 x 33 mm. The available heights for all diameters are 88 mm and 150 mm.
Convex End Rings
The Convex End Rings enable locking the end ring to the mesh with the new low-profile 2.0 mm or 3.0 mm Locking Screw.
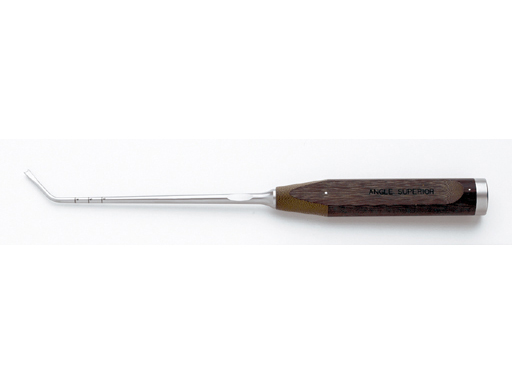
Forked Impactors
The Forked Impactors enable better maneuvering of the SynMesh construct into its final position. Three designs are available: angle lateral, angle superior, and straight.
Implant Holder, large and small with tips
The Implant Holder improves secure handling of the mesh and distributes the impaction forces over a larger area, reducing implant deformation during insertion.
SynCore Insert Trimmer
The Insert Trimmer is to be used with SynCore, which are cancellous bone inserts, to fill the interior of the SynMesh Spacer. The inserts can be stacked and/or trimmed to the desired lengths.
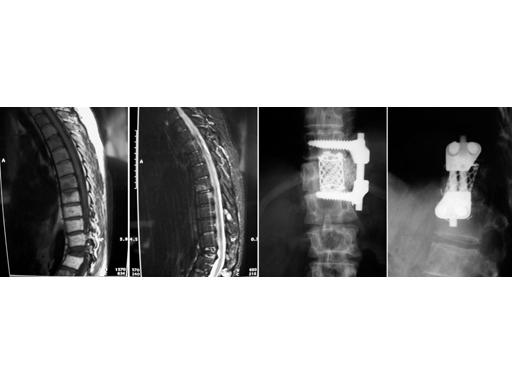
39-year-old woman, pathological fracture in L1 due to metastasis of breast cancer. Synmesh combined with Ventrofix.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.