Expert Tibial Nail System (ETNS)
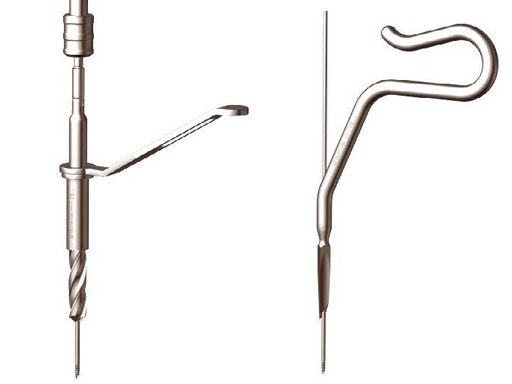
The Expert Tibial Nail System (ETNS) is an intramedullary nailing system which covers the indications of the PTN and of the UTN/CTN, plus more distal and more proximal indications. Additional to the standard static and dynamic locking options, the ETNS features multi directional locking options in the distal and proximal part of the nail. The ETNS has a 10 bent (8 plus 2) which eases nail insertion and extraction, and gives the nail a better anatomic position in the intramedullary canal. The Expert Tibial Nail System features cannulated nails in six different diameters from 8 mm to 13 mm and solid nails in three different diameters from 8 mm to 10 mm. Both are available in 15 lengths from 255 mm to 465 mm. All nails will be offered unsterile and sterile. The open instruments have been modified and feature a 12 mm Cutter, Straight Awl and Drill.
The End Caps have a long tip to help guide the End Cap into the top of the nail, making it easier to insert. Furthermore, the End Caps have a lead-in design so when inserted, the End Cap automatically aligns with the top of the nail. The End Caps are cannulated for use over a guide wire. Available lengths are 0, 5, 10, and 15 mm.
Locking options for the Expert Tibial Nail System
ETNS handling test
Intramedullary nailing today is still the method of choice in the treatment of tibial shaft fractures. Various nailing systems from different manufactures generally differ only in the details of their features that are on offer to the surgeon. For many years the AO has been advocating the application of the various designs of UTN. As the published literature of the last few decades shows, the UTN reliably stabilizes diaphyseal fractures of the tibia, however, in the meantime more innovative and user-friendly products have been introduced to the market.
Currently, a main goal of the modification and development of new nailing systems is, partly for commercial reasons, to make available a safe, modular system with which shaft fractures of the tibia, femur and humerus can be treated. This should include the possibility to utilize the same standard set, possibly with additional instruments, to implant the various types of nails. Obviously it is a basic condition that this system fulfils the technical and clinical requirements of intramedullary nailing osteosynthesis.
The recently developed Expert Tibial Nail System (ETNS) is, so to speak, the first module in a new product range from Synthes Inc. for the treatment of fractures of the long bones. The various innovative locking options, whereby the most proximally positioned screw can be locked at a fixed angle by insertion of an end cap, have broadened the range of indications both proximally and distally in comparison with the UTN.
Modifications have been made to the nail design based on results from the first handling test indicating that difficulties were still arising. The second ETNS handling test started in August 2004. Ten trauma centers in six different countries (D, A, CH, GB, NL, SF) participated in this prospective handling test.
Administrative planning and data collection was handled centrally by AO Clinical Investigation and Documentation (AOCID) in close collaboration with Synthes Inc. After a short preparatory period, 190 patients (118 male, 72 female) were successfully recruited to the handling test within a period of 11 months. The female patients were on average 9 years older than the male patients (average age 40.7 (male): 49.7 (female) years). There are age peaks between 20 and 30 years of age and 40 and 50 years of age.
The 12 week follow-up was performed for 154 patients (81% follow-up rate). The follow-up evaluation took place on average 91 days after the operation. In only three cases was a secondary dislocation of the fragments described at this time. These three patients all showed delayed fracture healing after complex diaphyseal fracture. In 88% (n = 136) of the cases available to follow-up, fracture healing is described as ongoing. Delayed union was observed in 18 patients. Nine of these patients had presented initially with an open fracture. One case of delayed healing was observed in a 54-year-old woman who had sustained bilateral fractures and posttraumatic paraplegia. In another case, delayed healing occurred in conjunction with wound infection. In the majority of these cases, the treating surgeon chose to wait before intervening. Dynamization of the nail was only performed in three cases
Definitive evaluation is expected to take place after completion of the 1-year follow-up assessments in spring 2006. This will provide us with the final results with regard to fracture healing, reduction, axial alignment, any complications, necessary revision operations, and an evaluation of the handling of the ETNS. It is planned to summarize the results in a publication.
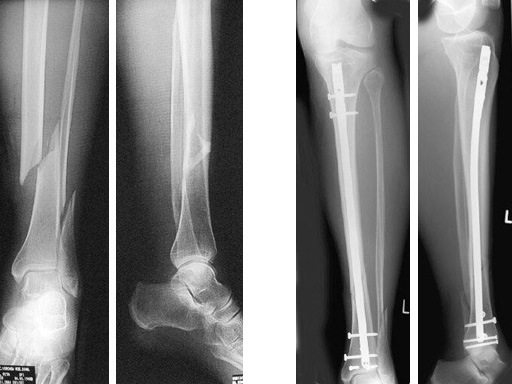
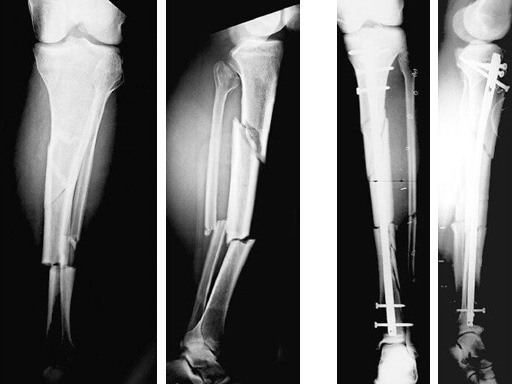
64-year-old woman with an open distal tibial fracture extending into the pilon treated with ETNS.
Type III open distal tibial fracture with beginning compartment syndrom following a traffic accident.
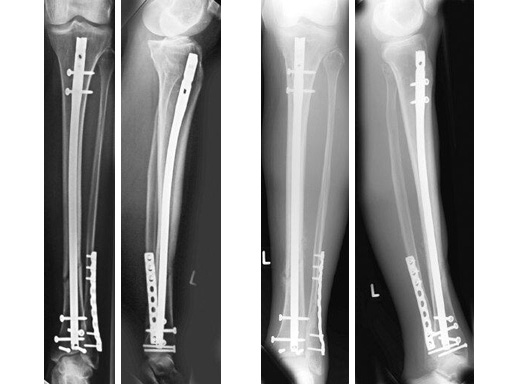
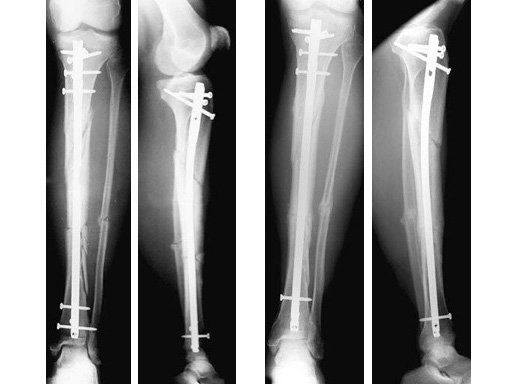
47-year-old man with closed 42-C3 fracture following a sports accident treated with ETNS.
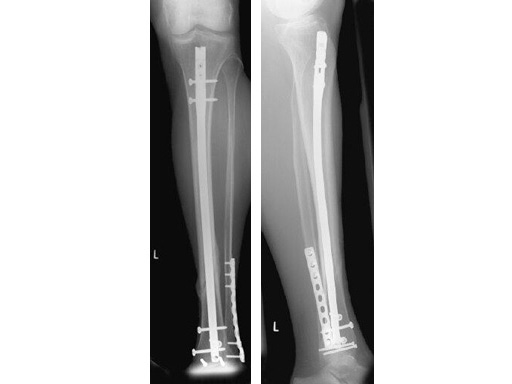
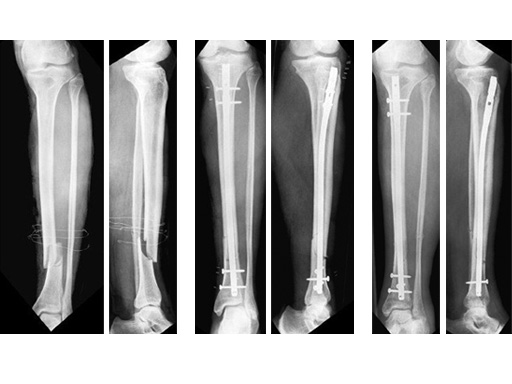
60-year-old man with a type II open 42-A2 fracture following a working accident.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.