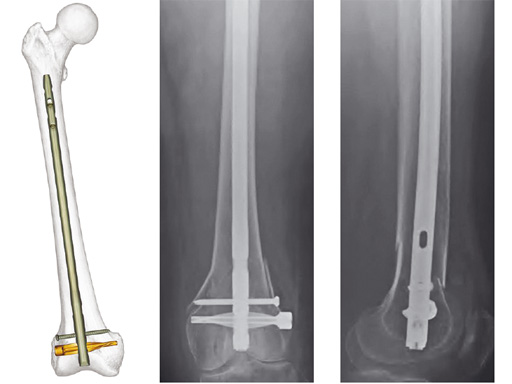
Expert Retrograde / Antegrade Femoral Nail (R/AFN)
The R/AFN is a cannulated intramedullary nailing system for the treatment of fractures of the distal femur and/or diaphyseal fractures in which a retrograde approach is indicated. The R/AFN also enables fixation of fractures of the femoral shaft with an antegrade approach through the piriformis fossa.
The R/AFN has a universal design for the right or left femur with an anatomic bend (1.5 meter radius of curvature). The locking options include spiral blade and standard locking. Proximal locking holes are optimized for the lengths of the nail. The use of a locking end cap secures the spiral blade or the most distal locking screw in place, creating an angular stable construct which is advantageous for fixation of fractures involving the femoral condyles.
The R/AFN is available in shaft diameters from 9 mm to 15 mm and lengths from 160 mm to 480 mm, in 20 mm increments. Lengths from 160 mm to 280 mm are designed for retrograde insertion only. Lengths from 300 mm to 480 mm can be used for both antegrade and retrograde insertion. 5.0 mm and 6.0 mm Ti locking screws are available with a T25 StarDrive recess.
A newly designed instrument set permits a more percutaneous technique and less tissue stripping. Compared to the Distal Femoral Nail (DFN), the R/AFN offers additional locking options, allowing also an antegrade approach.
Percutaneous Insertion Handle for LFN and R/AFN for obese patients
For soft tissue clearance in very obese patients, the standard insertion instruments may be too short, as the barrel of the standard insertion handle measures only 44 mm in length. Therefore, a longer barrel for the percutaneous insertion handle was designed with an increased length of 144 mm. The added length necessitates a longer mating connecting screw and a longer 5.0 mm hexagonal screwdriver to engage the LFN/R/AFN locking mechanism.
Because of its length a more proximal skin incision than is typically used is required. By concentrating on a more proximal skin incision in this obese population one might avoid the necessity of lengthening the skin incision and deep soft tissue dissection with the existing jig.
This additional handle should not be used on a normal nonobese patient as the added length of the arm may impinge on the soft tissues making the insertion more difficult.
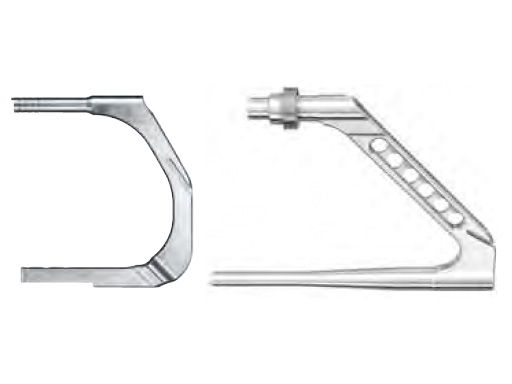
R/AFN Arm for Proximal Aiming Device
A proximal aiming device (PAD) enables surgeons to lock the nail distally and proximally completely without fluorescent x-ray. After determination of the nail length the surgeon must calibrate the proximal aiming device to the proposed nail. The proximal guide part leads to the correct location and orientation of the locking holes of the nail. For drilling and screw placement, the proximal guide is used in the same way as a normal aiming arm. Compared to the free-hand technique, the PAD offers a simple and effective solution for proximal locking of short nails.
For the short R/AFN (160200?mm), a new proximal aiming arm enables distal and proximal locking. It consists of a long aiming arm which is mounted onto the R/AFN insertion handle. The arm features drill holes for the proximal interlocking. It also accepts two inserts for distal interlocking, one for distal standard locking and one for distal spiral blade locking.
An aiming sleeve 12.0/8.0?mm with crosshairs, length 188?mm is used to check if the nail locking holes are still in line with the aiming arm holes.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.