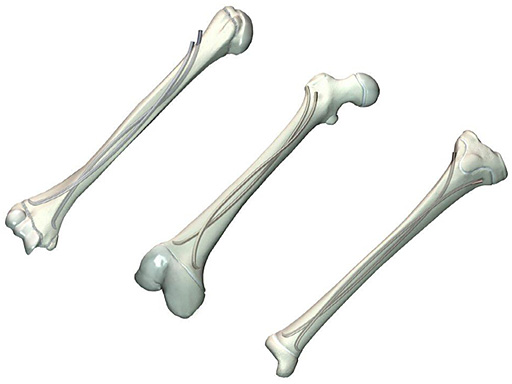
Elastic Nail in Titanium (TEN) and Steel (STEN)
Titanium Elastic Nail (TEN)
The Titanium Elastic Nail (TEN) is now also available in 1.5 mm diameter and 300 mm length.
Stainless Steel Elastic Nail (STEN)
Some surgeons routinely prefer a stainless steel implant and in some cases a more rigid osteosynthesis is required. Therefore, the AO has now developed a steel elastic nail with the same features as the TEN. The Stainless Steel Elastic Nail (STEN) is indicated primarily for managing diaphyseal and metaphyseal fractures in adolescents or heavy children. The STEN offers increased strength over the equivalent size TEN, but at the expense of decreased elasticity as it is a more rigid implant. The exact indications depend on the patients age as well as the type and site of the fracture.
The STEN is available in diameters of 1.5–4.0 mm.
The existing Titanium Elastic Nail (TEN) instrumentation can be used.
End Cap for elastic nails
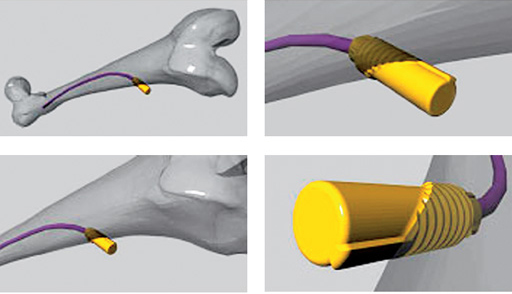
The End Cap is a new implant developed to enhance safety of the Titanium Elastic Nails (TEN) and Stainless Steel Elastic Nails (STEN) with indications where loss of axial stability/shortening is a potential problem/complication. This problem can be seen especially in completely unstable fractures, such as long oblique and spiral fractures, including fractures with a third fragment. One of the reasons for this problem is the backing-out of the nails at the entry point, especially when the correct biomechanical properties are missing. The End Cap simply stops the movement of the nail in the entry-point, especially in the backing out direction. As the longitudinal forces on the nail have a counter force in the End Cap the stability of the bone/implant interface in the fracture zone is enhanced too. The initial intention of using the elasticity of the nail to act as a spring in the fracture zone is regained as the nail is no longer slipping away.
The End Cap itself does not need any additional preparation, except when soft tissue does not allow it to be pushed down on the bone surface directly. The End Cap is easily applied directly over the end of the TEN or STEN after its cutting and final insertion. By screwing in the End Cap the insertion hole of the TEN/STEN this hole slightly enlarged and the End Cap is fastened in the bone by its thread.
The effectiveness of the End Caps has been proven in mechanical tests enhancing the push-out resistance of the Titanium Elastic Nails through the insertion hole by five times and more. An additional advantage was observed during removal. By removing the End Cap the insertion hole of the nails is opened automatically to a slightly bigger diameter and the nails are then easily grabbed and taken out.
The End Cap should be used for all unstable fracture patterns, where the use of the TEN/STEN is indicated. The End Cap is not intended to enlarge the indications for the ESIN technique, but to enhance the safety of its use. There is no contraindication against the use of the End Cap except in situations where the enlargement of the insertion hole might not be acceptable (very small entrance points).
In summary, the indications are unstable fractures of the femur or tibia in older children. The End Caps are designed for nails with a diameter between 3–4 mm.
First Clinical Experience with End Caps in Elastic Stable Intramedullary Nailing of Femoral Shaft Fractures in Children
Steffen Berger, Laurent Audig, Theddy F Slongo
Introduction During the last two decades, elastic stable intramedullary nailing (ESIN) has become the method of choice for internal fixation of femoral and tibial shaft fractures in children of 414 years of age. In the lower extremity, ESIN treatment may be complicated by loss of reduction following push-out of the nails at the entry site especially in unstable femoral shaft fractures. The rate of this complication, nail migration with subsequent soft-tissue and skin irritation was reported to be as high as 512 %. A technically simple method to achieve secure locking of ESIN was not available so far. An end-cap system for ESIN that is now available which locks the nails at the entry point was evaluated clinically. The end caps are equipped with a self-cutting device and are put over of the cut ends of the nails like a hollow screw that is fixed in the cortical bone at the nail entry site.
Methods 34 femoral shaft fractures in pediatric patients were treated by ESIN and end caps at the Department of Pediatric Surgery in Berne from January 2005 to January 2009. Fractures of an unstable type and higher weight or older age of the children were considered as an indication to add end caps to ESIN. Results were evaluated as to applicability of the end-cap system, fracture type, instrumentation stability, and fracture healing, and return to activity by analyzing patient charts, x-rays, and questionnaires including follow-up data.
Results 8 girls and 26 boys with a mean age of 105 38 months (range 415 years) were treated with ESIN and end caps. Nails of 2.54 mm diameter were used (2.5 mm: 1 case; 3.0 mm: 19 cases; 3.5 mm: 10 cases; 4.0 mm: 4 cases). There were 18 spiral fractures, 11 oblique fractures, 3 transverse fractures, and 1 pathological fracture. 32 fractures were nailed in a retrograde direction, 1 was nailed antegrade, and 1 was nailed with one antegrade and one retrograde nail. The majority of fractures extending from the proximal to the middle third of the femur shaft, only three fractures affected the distal third of the femur. A third fragment was found in 7, multiple fragments were present in 2 children. All fractures were stabilized sufficiently with ESIN and end caps, in 1 patient one end cap was loose and thus not functional. Loss of reduction was observed in none of the patients. Leg-length difference > 1 cm resulted in none of the patients at the last available follow-up. Varus/valgus deformity of 10 resulted in 1 patient after an additional trauma, an axial angulation <10 was seen in 3 patients postoperatively. The mean interval between operation and removal of the implants was 5.6 months. Removal of the end caps and nails was rated simple and uncomplicated in all cases (28/34 removed so far).
Discussion The use of end caps avoided postoperative instability in all cases of pediatric patients with femoral shaft fractures, even in heavier, older patients and with instable fracture types in a cohort treated by a nonselected pediatric surgical staff (eight different surgeons). To maximize stability of ESIN-instrumented unstable fractures, end caps require properly placed nails that are correctly bent. It is essential to cut the nails to a correct length at the entry site to ensure adequate anchoring of the nails in the caps and anchoring of the cap in the cortical bone respectively. A special bevelled impactor is mandatory for the final nail positioning. The position of the nail inside the end cap can easily be visualized since the caps are semiradiotranslucent.
End caps however, although offering additional stability to ESIN instrumentation, will not completely compensate for operative technical insufficiency concerning reduction or nail placement. They may contribute to axial and length stability especially in proximal spiral fractures, and in fractures that are unstable due to third fragments. It is assumed that end caps provide more comfort to the patient since no sharp nail ends will irritate the soft tissue above the entry points. End caps make the removal of nails easier since a canal is present in the cortical bone around the nail after removal of the cap and nails come out with less effort.
We conclude that end caps should only be applied by surgeons that are fully aware of all technical details and problems of ESIN. With this precondition, end caps might prove beneficial in a standard clinical setting.
Project: End Cap for TEN/STEN
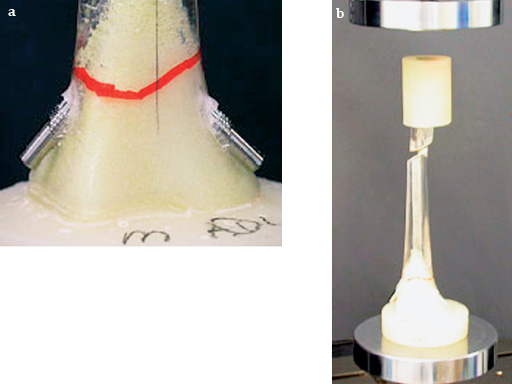
When using the TEN system for the stabilization of infant, diaphysial fractures in older and heavier children, the following effects, as shown in the picture of a mechanical laboratory simulation, should be avoided:
Risk of backing out with subsequent soft tissue irritation
Risk of sliding in
Idea
End Cap for TEN to ensure secure fixation relative to the bone.
Prototype
12.2002: presentation of first Synthes-Stratec prototypes and definition of clinical problem.
10.2003: invitation of ADI to be involved in the project.
To clarify the requirements, the clinical inputs have been discussed within the Development Service Group. The clinical inputs have been prioritized and further translated in to technical descriptions. In the following development phase we separated the needs in to features. This features have been combined in different arrangements and were checked within a 3-D construction program in terms of, placement, geometry, fit, eg, and have been discussed in the Pediatric Expert Group.
After construction and finalizing of the drawings the first prototypes have been discussed for production in the prototype team of ADIs Workshop. In parallel an alternative solution was worked out by Synthes. The produced End Caps and the alternative of Synthes have then been used for further mechanical testing in cooperation with the Research Service Group of the ARI and Synthes. The test was designed to clarify the following points: difference between using the two designed End Caps and not using an End Cap. The results were checked for distal and proximal migration of the Nail, displacement of the fractured bone model and eventual backing out of the End Cap. The End Caps were tested also in three different insertion angles in relation to the bone, 40, 45, and 50. The test was performed in artificial bone models. After mechanical testing and additional handling tests the Expert Group agreed to pursue ADIs solution because the design incorporated the following features: bidirectional drill tip which allows easy insertion in angles from 40 to 50 and no soft tissue irritation due to the oblique threat design.
Serial product
03.2004: after final testing, hand over of the first ADI prototype to Synthes Inc. for final product development.
Performance of the End Cap System
Biomechanical study of elastic stable intramedullary nailing (ESIN) in pediatric fractures.
Objective
Elastic Stable Intramedullary Nailing (ESIN) is a common method for treatment of pediatric femoral shaft fractures. Backing out of the nails at the insertion sites is a potential problem in unstable fractures of older children. To overcome this problem End Caps may be placed over the nail ends and screwed into the bone (see also page 16). This in vitro study investigated two aspects: (a) The effect of the End Cap insertion angle upon the End Cap push-out force and (b) the potential advantage of using End Caps with regard to backing out of the nails.
Materials/methods
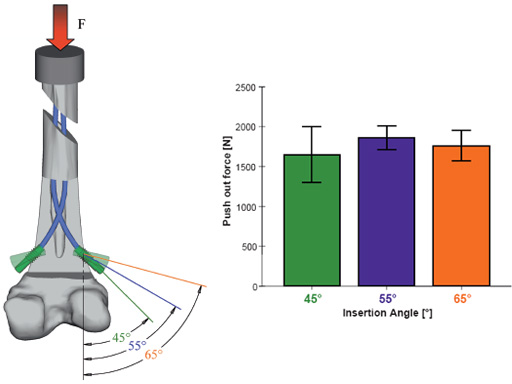
(a) Oblique midshaft defects of 1.5 cm lengths were created in artificial pediatric femurs to simulate completely unstable fractures. Fixation was performed with 3 mm Titanium Elastic Nails (TEN) and End Caps inserted at angles of 45, 55, and 65 to the long axis of the femur (n = 5 for each group). Specimens were shortened about 2 cm above the defect, embedded in a fixture to avoid proximal nail migration and placed on a materials testing machine for axial loading at a crosshead speed of 5 mm/min (Fig 1). Push-out force F and displacement were recorded.
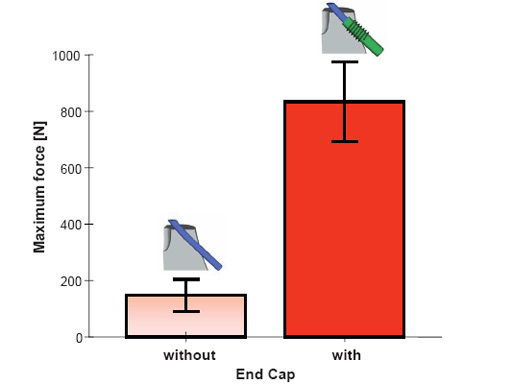
(b) An identical defect model was created in four small adult cadaveric femurs and treated with 3.5 or 4 mm TEN. Applying the same experimental methods, all femurs were first tested without and subsequently with End Caps inserted at 45. Maximum force was recorded until nail backing out occurred.
Results
(a) The End Cap insertion angle did not influence End Cap push-out force in the artificial bone model (P = 0.613).
(b) The force necessary to provoke backing out of the nails was up to 6 times higher when using End Caps compared to the condition without End Cap. This difference was statistically significant (P = 0.007).
Bend Awl
In order to use the TEN for treatment of simple clavicle fractures it requires an opening device for medial or lateral TEN insertion. The Bend Awl allows to properly open the clavicle from either side to enable adequate TEN insertion.
Case 1
Case of a 8-year old boy with a closed lower leg fracture after football game.
Case 2
(provided by Steffen Berger and Theddy F Slongo, Bern, CH)
Fracture of the right femoral shaft (32-D/5.2) in a 8-year-old boy.
Case 3
6-year-old girl, ski injury.
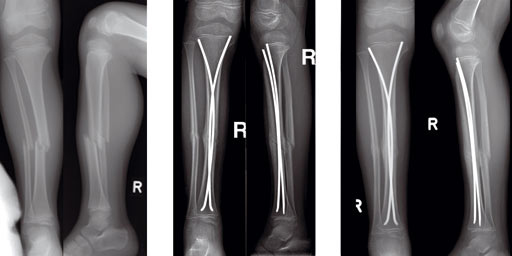
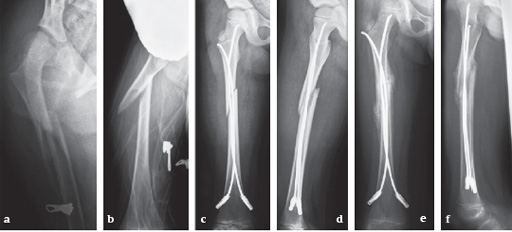
Fig 13a-b: X-rays show a long spiral fracture of the proximal third of the right femur (32D/5.1). Because of the age good indication for ESIN, but some danger of instability is possible.
Fig 13c-d: Postoperative x-rays. A closed reduction and fixation with 3.0 mm TEN was carried out with correct child-oriented alignment. For stability reasons two End Caps became uses. No signs of a shortening can be seen.
Fig 13e-f: AP and lateral view 6 weeks after surgery shows good callus formation and still a perfect alignment.
Full weight bearing was then allowed.
Case 4
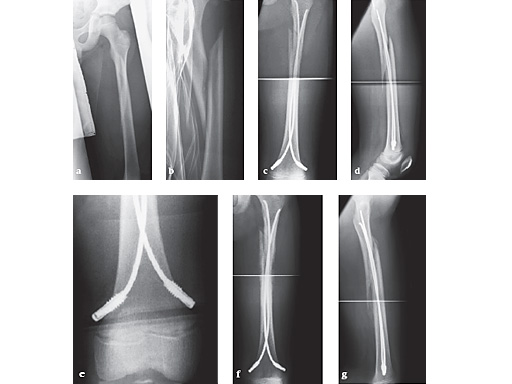
12-year-old boy; snow-board injury long spiral proximal femoral fracture (32D/5.1) primary indication for operation with ESIN and End Cap.
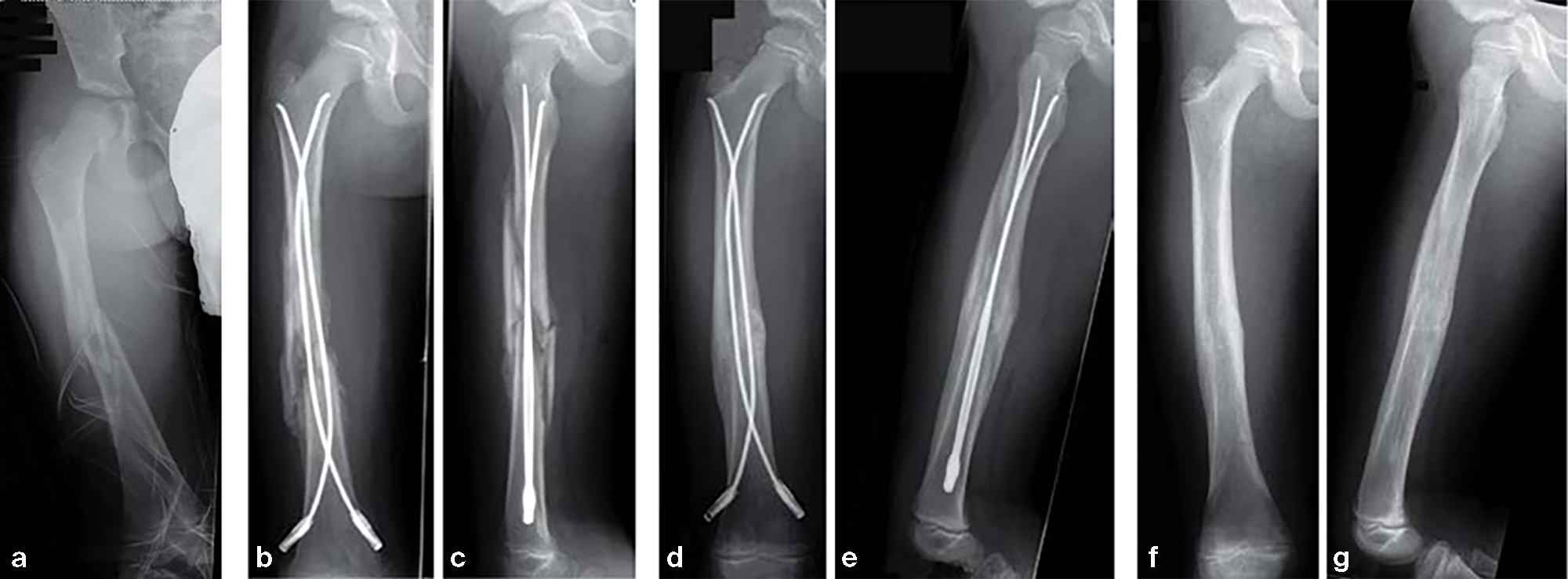
Fig 14a-b: Injury x-rays.
Fig 14c-d: Postoperative x-rays show a correct alignment and length. Fixation with 3.5 mm TEN and End Caps was performed.
Fig 14e: This detail view shows the correct positioning of the End Caps.
Fig 14f-g: 4 1/2 weeks postoperative a good callus and correct alignment was visible, full weight bearing was allowed.
Fixation options for the adolescent femoral fracture
Elastic Nailing for Childrens Fractures: How to Avoid Complications
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.