AO Research Institute Davos (ARI) helps drive precision medicine development targeting rheumatoid arthritis
The official kick-off took place on February 4–5.
The European Union (EU)—under a Research and Innovation Action (RIA)—recently gave a significant financial boost to a rheumatoid-arthritis-focused consortium of which the AO Research Institute Davos (ARI) is a member. Thanks to the EU, the FLAMIN-GO project will receive EUR 6 million to support its work to develop an organ-on-a-chip platform to mimic rheumatoid arthritis (RA) of the joint and test drugs in a personalized way. ARI will receive CHF 500,000 to support its work on the project.
The consortium, comprising 14 institutions in nine countries, brings together experts in the fields of rheumatology, material science, tissue engineering, nanotechnology, cell biology, and 3D modelling, in a cohesive, transdisciplinary, multi-sectorial approach taking on the challenge to drive RA personalized care.
"FLAMIN-GO will develop personalized treatments for rheumatoid arthritis through a platform that mimics in a LEGO-like building block approach, all the tissue affected by this inflammation: cartilage, synovial tissue, vascular system, and immune cells," explains ARI research scientist Tiziano Serra, a member of the Sound Guided Tissue Regeneration Focus Area.
Rheumatoid arthritis (RA) is an autoimmune inflammatory disorder, primarily characterized by synovial joint inflammation, affecting around 0.5–1% of the overall population (approximately 2,900,000 patients in the EU) and is more common in women than men (3:1). RA is a huge public health problem because, over time, it leads to permanent disability. There is no cure for RA but remission of symptoms is more likely when treatment begins early, according to FLAMIN-GO. However, approximately 40 percent of RA patients fail to achieve even 20 percent improvement in disease activity, with significant disability remaining in about a third of patients, and major work-related and social costs for patients and society.
In addition, 10-20 percent of patients do not respond to any current medication, pointing to considerable disease heterogeneity and the need for testing and developing new drugs. A further point related to RA heterogeneity is that there are no biomarkers of treatment response to individual drugs. So, a number of unmet needs still persist particularly related to response/nonresponse to powerful but expensive drugs. Conventional randomized clinical trials (RCT) may address some of these challenges, but they are time-consuming, expensive, and ethically doubtful, since many patients (currently about 40 percent, regardless of the modality of action) fail to achieve disease benefit, while being exposed to potentially toxic drugs. Thus, the rheumatology community has a need for development of an alternative strategy to deliver innovative trials.
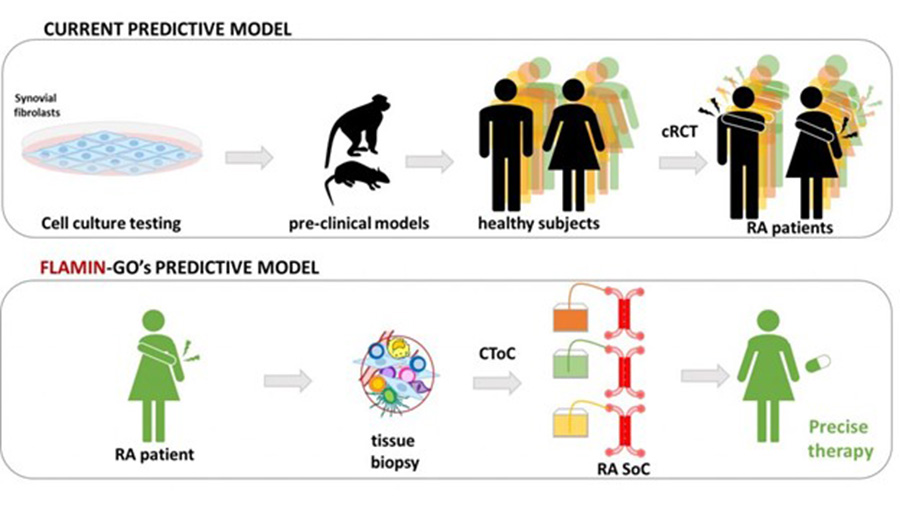
FLAMIN-GO’s mission is to develop a personalized next-generation synovia-on-chip (SoC) that, by effectively mimicking the complexity of a rheumatoid-arthritic joint, will permit patient-specific clinical trials-on-chip (CToC). This includes:
- Selecting the best on-market drug for each patient’s treatment, in order to obtain maximum benefits and reduce the risk of side effects.
- Enabling rapid discovery and testing of new therapeutic targets, contributing to determine a new drug development path
Serra explained ARI’s work on the FLAMIN-GO project.
"We will work on the osteochondral unit of this multi-tissues organ on a chip platform and on the hybridization of bioprinting technologies with complex microfluidic systems, which is still a challenge in terms of automation and scalability," he said.