Prophylaxis and treatment
-
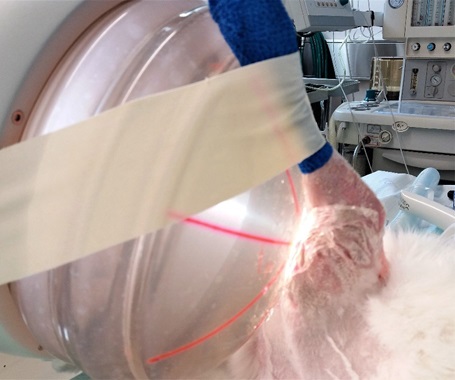
High-energy focused extracorporeal shockwave
High-energy focused extracorporeal shockwave as an adjunct to antibiotic treatment in fracture-related infection in rabbits.
Jan Pützler, University Hospital Muenster, Germany
A fracture-related infection (FRI) is one of the most feared complications in orthopedic trauma surgery. Despite best practice in prophylactic antibiotic therapy, infection rates still reach up to 5 percent in closed fractures and 30 percent in open fractures. Modern prophylactic approaches, such as anti-infective coatings on implants, might decrease the infection rate in high-risk situations such as open fractures. However, once an FRI is established, conventional treatment comprising of surgical debridement and prolonged systemic antibiotic therapy fails in approximately 10 to 30 percent of patients. Therefore, additional strategies are needed to improve treatment.
Focused high-energy extracorporeal shockwave therapy (fhESWT) improves fracture healing in cases of non-union. Additionally, it has direct antibacterial effects. However, at the present time, acute FRI is regarded as a contraindication for fhESWT due to fear of inducing bacteremia. fhESWT was evaluated as an adjunct to conventional treatment in a clinically relevant rabbit model of FRI.
In combination with antibiotic treatment, fhESWT lowered the bacterial burden 100-fold as compared to antibiotic treatment alone. This was most prevalent for the bacterial colonization on the implants (plate and screws). Alone, fhESWT had no relevant effect on bacterial reduction. No signs of bacteremia occurred in blood cultures taken pre- and post-intervention and in indicator organs (brain, heart, liver, spleen, lungs, kidneys). Thus, fhESWT appears safe and could be a helpful adjunct to conventional treatment warranting further investigation. -
Passive immunization
Pre-clinical evaluation of anti-autolysin passive immunization for infection of implant-associated osteomyelitis.
Edward M Schwarz, University of Rochester, United States
The project aims to prevent and treat bone infections caused by antibiotic-resistant organisms, specifically methicillin-resistant Staphylococcus aureus (S. aureus) (MRSA). To test the effectiveness of passive immunization with monoclonal antibodies (mAb) that have been generated against the glucosaminidase (Gmd) subunits of the S. aureus surface autolysin (Alt) protein, the project will be conducted as follow:
• cGMP large-scale production of anti-Gmd mAb for investigational new drug (IND)-enabling preclinical safety and efficacy studies
• In vivo pharmacokinetics and biodistribution studies in mice
• Assessment of therapeutic safety and efficacy of anti-Gmd mAb therapy in a murine model of one-stage revision surgery for fracture-related infections (FRIs)
A step-by-step process will be used to determine the maximally effective antibody for prevention of MRSA osteomyelitis in a series of preclinical tests.
A novel, quantitative one-stage septic revision murine model using a S. aureus-infected 4-hole femoral plate was developed (Yokogawa et al, 2018). The model allows quantitative assessment of intervention efficacy on day 14 post-infection by colony-forming units (CFU) count on the implants (primary outcome), longitudinal bioluminescent imaging (BLI), micro-computed tomography assessment of osteolysis, and histomorphometry of S. aureus abscess communities. Synergistic effects of adjuvant anti-Gmd passive immunization with parenteral antibiotics were found in pilot studies (Yokogawa et al, 2018).
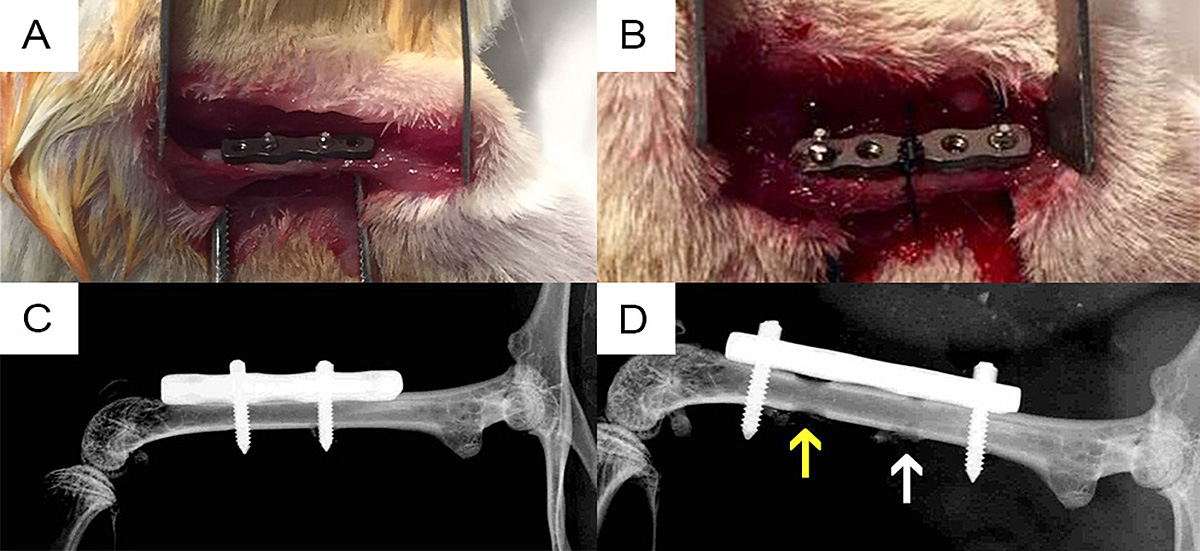
Figure: (A) Intraoperative photo of the RIS Mouse Fix Plate™ and internal screws prior to wound closure on day 0. (B) Intraoperative photo of the revised-implanted RIS Mouse Fix Plate™. (C) Postoperative day 0 X-ray of the RIS Mouse Fix Plate™ and internal screws, of which the distal screw was contaminated with 105 CFU of MRSA. (D) X-ray of the revised-implanted RIS Mouse Fix Plate™. Arrows indicate position of screws during the first surgery. Note that the hole of the contaminated screw (yellow arrow) is more profound than that of the sterile screw (white arrow) due to osteolysis.
-
A large-animal model for staged revision of chronic implant-related bone infection
Development of a large-animal model of chronic implant-related bone infection with staged treatment protocols based on best surgical and medical practice and evaluation of novel antibiotic-loaded thermo-responsive hyaluronic acid hydrogel as an adjunct to systemic antibiotic therapy.
Fintan Moriarty, Stephan Zeiter, David Eglin, AO Research Institute Davos (ARI), Switzerland
Edward M Schwarz, University of Rochester, United States
Stephen Kates, Virginia Commonwealth University Richmond, United StatesA fracture-related infection (FRI) is a devastating complication, especially when caused by antibiotic-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA). Despite an extensive treatment protocol that includes multiple surgeries and antibiotic therapy, the procedure is associated with relatively high failure rates. Currently, no large-animal model recapitulating this scenario is available. Consequently, new innovations cannot be tested in an appropriate model. Therefore, the first aim of the project was to establish a large-animal model for failed treatment of a chronic orthopaedic-device-related infection in order to serve as a testbed for future interventional strategies.
The model
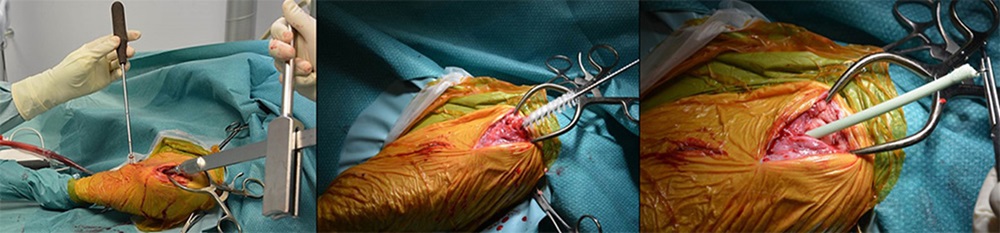
We use adult Swiss Alpine sheep, who receive a commercially available intramedullary nail in the tibia, fixed in place with a locking bolt. A localized inoculum of either a methicillin-sensitive or methicillin-resistant Staphylococcus aureus (MSSA, MRSA, respectively) is placed adjacent to the nail and a chronic infection is allowed to develop over 8 weeks. Treatment commences with implant removal, debridement and temporary placement of an antibiotic-loaded cement spacer (see figure). Antibiotics are delivered systemically intravenously for 6 weeks. A definitive fixation procedure involves further debridement and placement of a definitive intramedullary nail fixation. In this model, conventional treatment, as just described, achieves an approximate 50 percent treatment success rate, which closely mimics failure rates in humans with chronic MRSA infection.
The AO Research Institute Davos (ARI) intervention: thermo-responsive hyaluronic acid hydrogel
ARI has developed a bioresorbable, thermo-responsive hyaluronan hydrogel loaded with the antibiotics gentamicin and vancomycin that can act as an adjunct to systemic therapy in the treatment of MRSA infections. To provide a robust test of efficacy, this sheep model of a chronic MRSA infection was used to assess efficacy of the hydrogel. To date, the hydrogel has been shown to be superior to gold standard treatment when applied at both revision and definitive fixation procedures.
The next steps in this project will look to determine if the bioresorbable hydrogel can be applied in a direct renailing and maintain this treatment success rate.
-
3-D-printed anti-infective scaffolds
Development of custom-made 3-D-printed antibiotic-impregnated cement spacers for treatment of fracture-related infections.
Hani Awad, Center for Musculoskeletal Research, University of Rochester Medical Center, Rochester, United States
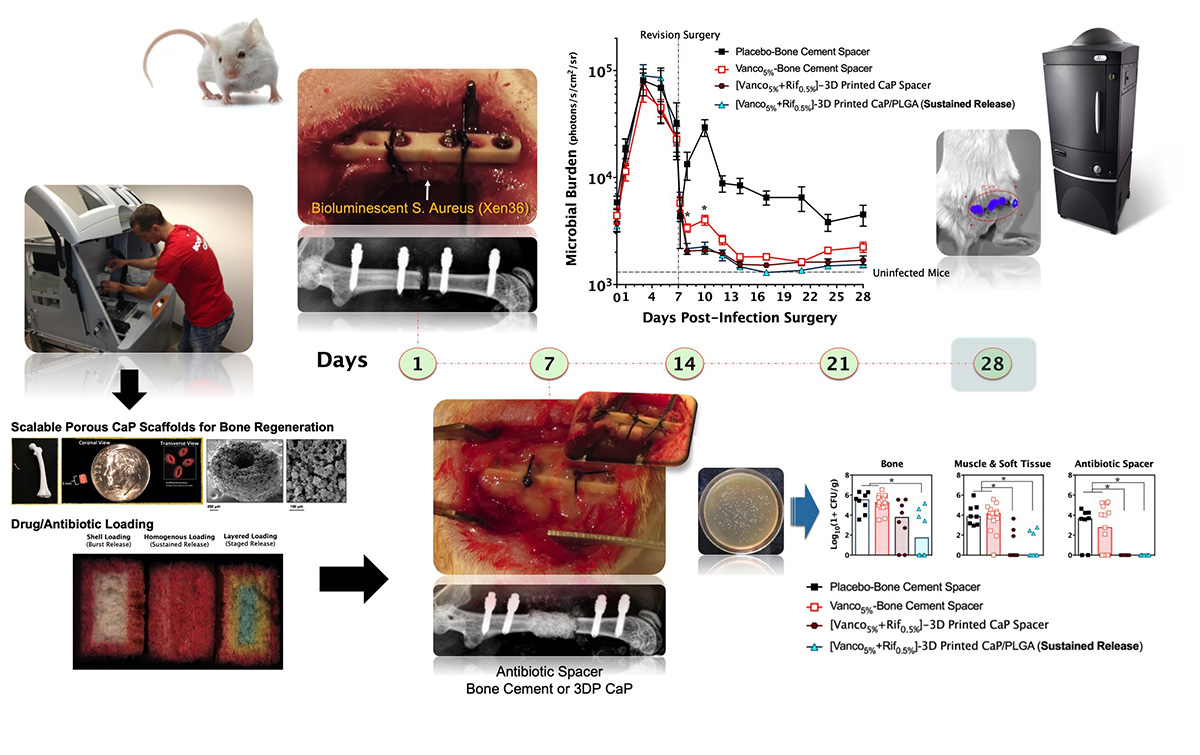
This project has led to the development of innovative strategies for adapting low-temperature 3-D printing technology to fabricate osteoconductive calcium phosphate (CaP) scaffolds for applications in preclinical small and large animal models of bone regeneration and infection. Particle-binding 3-D printing technology has been adapted to create CaP scaffolds for concomitant, local delivery of multiple antibiotics to significantly improve the outcomes of established implant-associated bone infection (osteomyelitis) in a mouse model as compared to the current clinical practice of using antibiotic-laden poly(methyl methacrylate) (PMMA) spacers. This technology has translational potential in medical image-guided reconstruction of massive bone loss in scenarios involving extremity bone and craniomaxillofacial trauma or infections.
Through fundamental and translational studies, the following results have been achieved.
1. Optimize the formulation and process of low-temperature 3-D printing of CaP scaffolds for reproducible accuracy, mechanical strength, and biocompatibility, and demonstrate the osteoconductive and regenerative potential of these 3-D-printed CaP scaffolds in a critically sized murine femoral defect.
2. Develop a novel mouse model, with an established bone infection in association with a fracture fixation plate, which can accommodate an antibiotic spacer after infected tissue debridement. Such a model could recapitulate the clinical features of implant-associated osteomyelitis and enable investigation of local and systemic antibiotic therapies through both longitudinal and end-point quantification of the infection and changes to the bone.
3. Adapt low-temperature 3-D printing of vancomycin- and rifampin-laden CaP scaffolds for sustained bactericidal delivery. This “next-generation” spacer technology with dual-antibiotic delivery significantly reduce bacterial burden and osteolytic bone loss of an established implant-associated bone infection as compared with vancomycin-laden PMMA, which is considered to be a clinical standard of care.
4. Complete a high-throughput screen (HTS) of an FDA-approved drug library containing 853 drug candidates to specifically identify candidates that were not only bactericidal against Staphylococcus aureus (S. aureus) normal colony phenotype (NCP) and biofilm but also against small-colony variant strain (SCV). Then, HTS hits were further validated and characterized for their antimicrobial efficacy against wild-type S. aureus strains, antibiofilm activity, resistance frequency, and human-cell cytotoxicity. This approach has led to the identification of the therapeutic potential of sitafloxacin to be repurposed for treatment of chronic S. aureus infections associated with SCV and/or biofilm growth states.
5. Design a biocompatible and osteoconductive CaP spacer (CaPS) to achieve sustained release of antimicrobial drugs (rifampin and sitafloxacin) that are effective against biofilm and SCV and investigate the efficacy of antibiotic-laden CaPS in the management of implant-associated osteomyelitis and bone healing in a single-stage revision approach with complete hardware exchange. The results of the study show that osteoconductive 3-D-printed CaPS incorporate with these antimicrobials, demonstrating more efficacious bacterial colonization outcomes and bone growth in a single-stage revision in comparison to gentamicin-laden PMMA, requiring a two-stage revision.
The pillars of the CPP Bone Infection
Host-pathogen interaction
Learn about how S. aureus adapts to the environment of an implant-associated infection.
Diagnosis
Read about the development of new tools to assess implant-associated infections.
Prevention
Explore how the CPP Bone Infection is running its surgical site prevention program.
Publications
Check the publications of the CPP Bone Infection