CONDUIT™ Cervical Cages—Part of the CONDUIT™ Interbody Platform
Maarten Spruit
The Cellular Titanium Cervical Cages are intervertebral body fusion devices intended for use for anterior cervical interbody fusion in skeletally mature patients with cervical disc degeneration and/or cervical spinal instability, as confirmed by imaging studies (radiographs, computed tomography [CT], and magnetic resonance imaging [MRI]), which results in radiculopathy, myelopathy, and/or pain at multiple contiguous levels from C2-T1.
The Conduit Cellular Titanium Cervical Cages can also be used with supplemental anterior fixation systems that have been cleared for use in the cervical spine. The cages are designed for use with autogenous and/or allogeneic bone graft to facilitate fusion.
The proprietary design of the CONDUIT cages with the interconnected porous structure that mimics cortical and cancellous bone, implant surface features, and implant stiffness is specified extensively here.
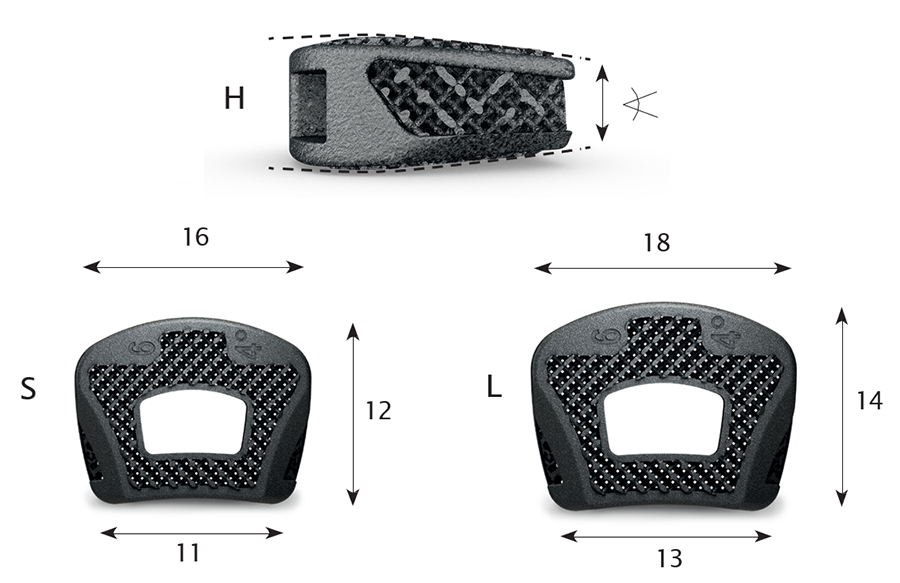
To allow optimal fit for patients and to address anatomical requirements to correct spinal alignment and sagittal profile, the CONDUIT Cervical System is offering various footprint sizes (12 x 16 mm and 14 x 18 mm), lordotic angles (4° and 8°), and heights (4−10 mm in 1 mm increments). They come in asymmetric design dome-shaped cranial surface and flat caudal surface, the width and depth of the vertebral end plate is in ratio of 1.3–1.4, the anterior rim is larger than the posterior width, and the lateral wedge design allows maximal contact of the uncovertebral joint (Fig 2).
Due to the implant's porous structure the height of the implant is slightly (0.25 mm) higher than the trial.
The texture and architecture of the CONDUIT Implants facilitate fusion by acting as cellular titanium scaffold. The porosity of the implants (approximately 80%) provides excellent clinical imaging on radiogarphs, MRI, and CT and is leading to a Modulus of Elasticity like bone.
The cages are designed for central grafting.
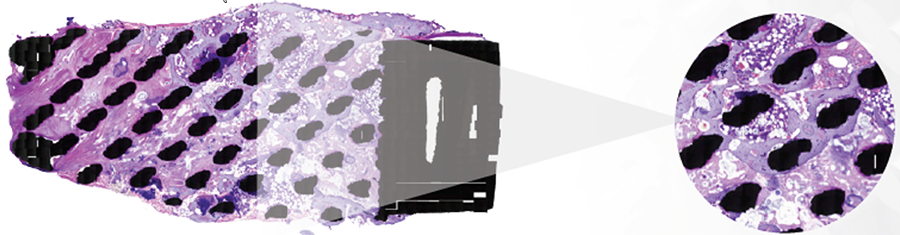
An implant retrieval case study (n = 1, cervical system explant 2 years postoperatively) displayed extensive bone in-growth end plate-to-end plate, indicating mechanical loading throughout the implant and mature, lamellar bone, and healthy bone marrow in direct contact with the titanium scaffold without fibrous tissue interface formation.
Conclusions
The Conduit Cervical cage is a 3D printed titanium cage design that allows graft and facilitates anterior cervical interbody fusion surgery. The cellular titanium material as such is attractive to bone, and the cage line addresses individual anatomical variety and creates optimal end plate to cage interface.
Case 1
(kindly provided by Maarten Spruit, MD, St Maartenskliniek, Nijmegen, Netherlands)
A 70-year-old man presented with neck pain, but predominantly arm pain bilateral L>R for 4 years. The patient experienced loss of sensation in C6 dermatome L. The conservative care and pain management was not effective. A medical examination revealed the following:
- Multidirectional decreased range of motion
- Spurling test positive L
- Loss of sensation C6 L
- Symmetrical reflexes, no hyperreflexia
- No pathological reflexes
- Romberg negative
- Adson negative
- No shoulder impingement
- Numerical Rating Scale (NRS) neck 3 and arms 7
- Neck Disability Index (NDI) 28
- Electromyography: radiating signs C6, but no denervation
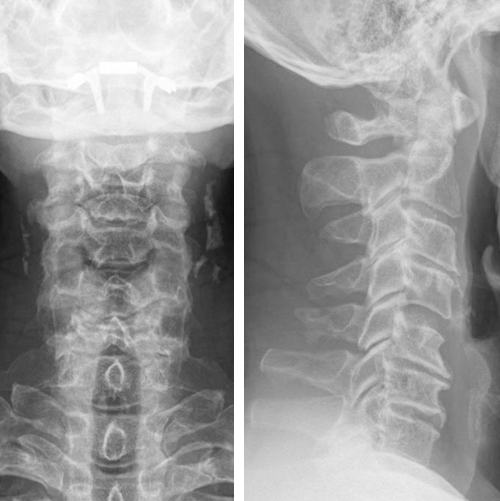
The radiograph indicated:
- Degenerative discs and spondylosis C3-4-5-6-7
- No translational instability on flexion-extension
- Loss of lordosis (Fig 4)
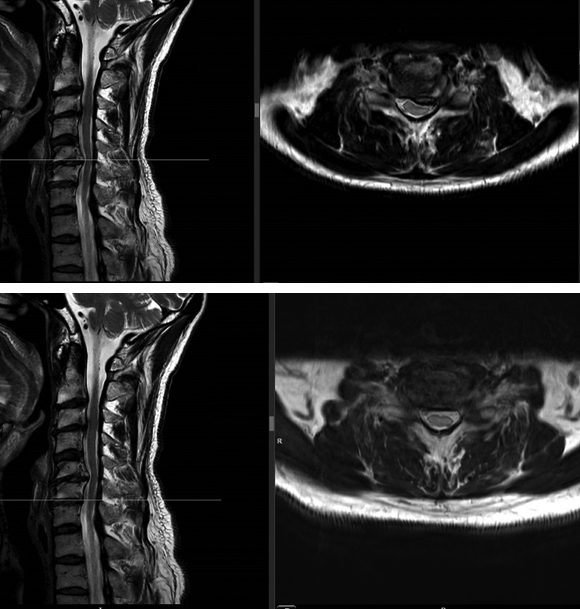
A magnetic resonance imaging demonstrated a cervical spinal stenosis with cord compression C5-6 L, foraminal stenosis C5-6 L, and C6-7 bilateral (Fig 5).
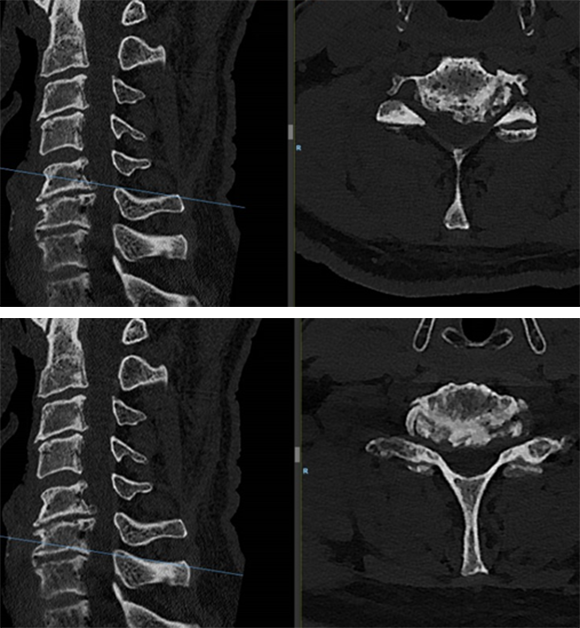
A computed tomographic scan showed large posterior osteophytes C5-6 and spondylosis anterior (Fig 6).
The diagnosis made was cervical radiculopathy L>R C5-6 and C6-7 and non-symptomatic spinal cord compression C5-6.
Surgical treatment
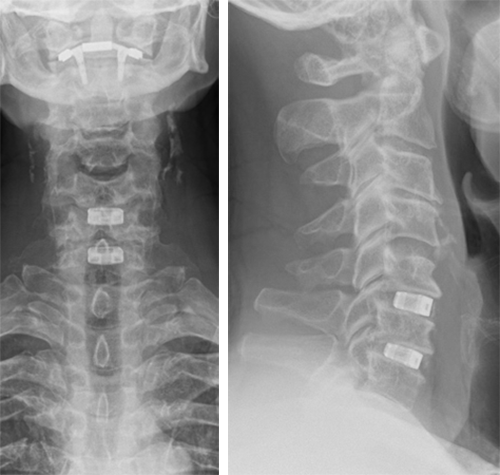
The patient underwent anterior decompression and fusion C5-6-7 with Conduit stand-alone cages C5-6-7. Restoration of lordosis C5-7 (Fig 7).
Follow-up at 3 months
The patient had an uneventful recovery, with neither neck nor arm pain, but with good residual range of motion. He had persistent loss of sensation C6 dermatome L.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO Foundation”
The brands and labels “approved by AO Technical Commission” and “approved by AO Foundation”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.