UNIUM™ Small Bone and Trauma Power Tool
Andrew Sands, Michael Swords
The UNIUM™ Power Tool was designed for increased reliability, efficiency, and comfort of use for an enhanced surgical experience and improved outcomes. It is intended for use in traumatology and orthopedic surgery that may include drilling, reaming, burring, screwing, tapping, sawing, and setting pins and wires.
The UNIUM Small Bone Power Tool consists of a modular handpiece for general use and a standalone reciprocating saw handpiece for cardiothoracic surgery (specifically for sternotomies). The strength and durability of both UNIUM Handpieces is enhanced by a high-quality PEEK housing and a stainless-steel coupling.
The system covers the following surgical areas: lower extremities (including intramedullary reaming but excluding acetabular reaming), pelvic, upper extremities, spine, sports medicine, and cardiothoracic.
UNIUM is a next generation small bone power tool that is approved by the AO Technical Commission and complies with the required standards for operating room use and addresses the needs of today’s healthcare professionals.
The system received AO TC approval for veterinary use in 2022.
Clinical problem
The use of power tools is closely associated with modern orthopedic surgery. These tools have advanced surgery by allowing surgeons to work efficiently and accurately. Pneumatic and electric power tools which require cords have been largely replaced by battery-operated devices because of their unconstrained and more comfortable use.
The battery-driven power tool Colibri II/Small Battery Drive II manufactured by DePuy Synthes has been available since 2012 (it is sold under the brand name Colibri II in Europe, Middle East, Africa, Asia Pacific, and Latin America, and under the brand name Small Battery Drive II in the US and Canada). Surgeons have long appreciated its ergonomic design, ease of use, and the high capacity delivered by the 14.4 V Lithium-Ion battery pack.
Since the initial launch of the Colibri II/Small Battery Drive II, the small bone surgery requirements have been evolving and increasing in complexity. In response, there is a need for improved devices which address the following essential demands:
- Lightweight and compact design for precise handling and low hand fatigue.
- Power, torque, and speed to match the broad range of applications in the small bone segment as well as heavy duty surgery.
- The battery capacity should be sufficient to cover the whole surgery to avoid intraoperative battery change. The battery must deliver consistent performance.
- Long lifetime and reliability of the system.
The Foot and Ankle Expert Group together with DePuy Synthes have developed an innovative power tool system that takes advantage of the latest technologies to fulfill these demands.
Solution
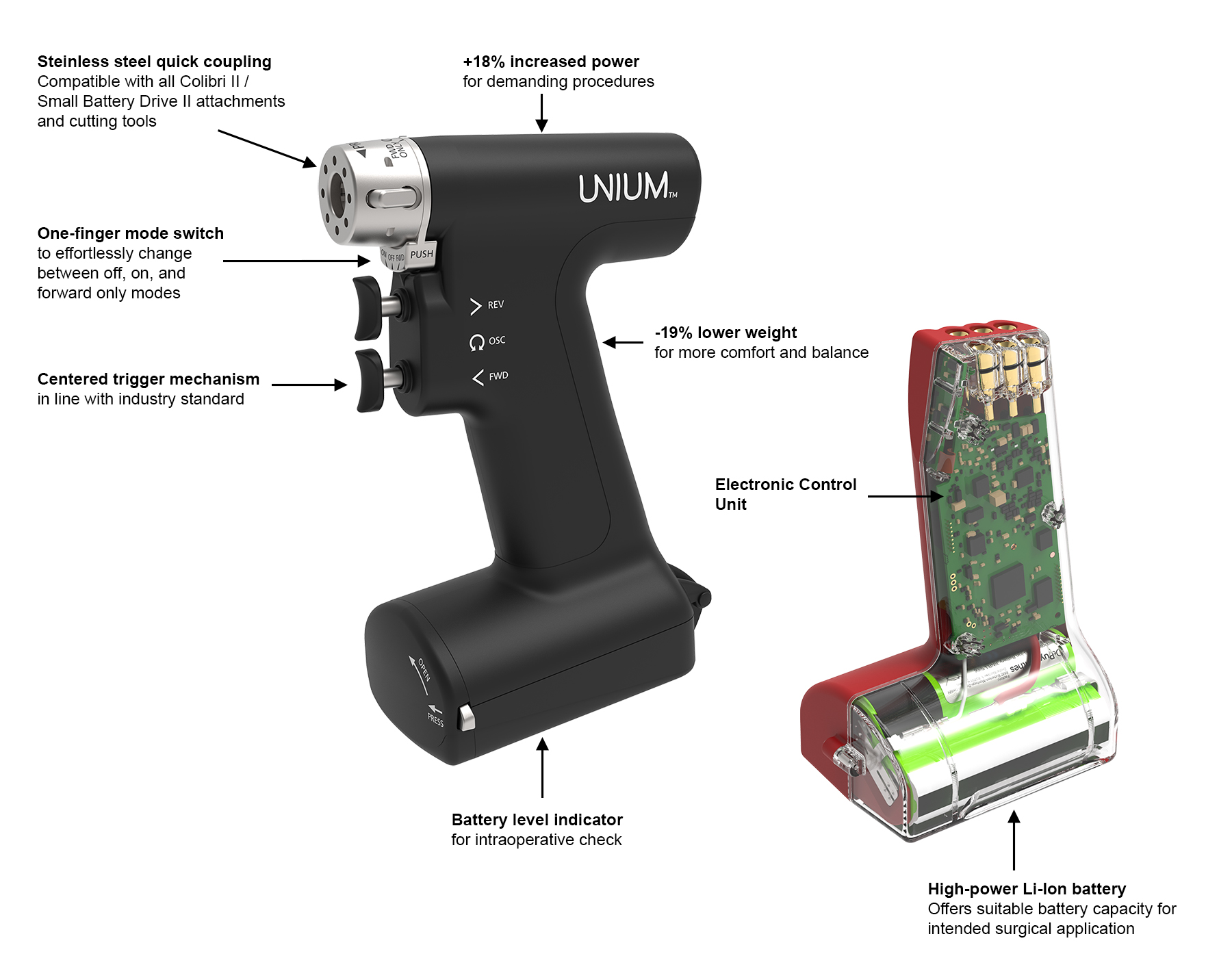
The UNIUM Handpiece is more powerful (100 W) than the Colibri II/Small Battery Drive II (85 W), although it weighs 175 g/0.39 lbs less (Fig 1). The increased power is expected to be beneficial for the performance in dense bone conditions (eg, in hindfoot arthritic bone and dense subtalar bone) and for reaming of long bones.
The trigger mechanism of the modular handpiece is different to that of the Colibri II/Small Battery Drive II and is in line with current industry standards. The lower trigger is for forward operation and the upper trigger for reverse operation. The oscillation mode is activated by pressing both triggers simultaneously. Additionally, the handpiece can be put in “forward only mode”.
The UNIUM Small Bone Power Tool is powered by an innovative Power Unit technology that incorporates the Electronic Control Unit and Li-Ion battery (Fig 2). These temperature sensitive parts are not exposed to steam sterilization, which extends the lifetime, reliability, and sustainability of the platform.
The UNIUM Modular Handpiece is compatible with all existing Colibri II/Small Battery Drive II attachments (Fig 3) (except for the K-wire attachment which has been updated as noted below), UBC II charger, and cutting tools for versatile use. UNIUM has a K-wire attachment with a range of Ø 0.6–3.2 mm. There is an increased output speed of up to a maximum of 1,700 rpm.
The standalone UNIUM Reciprocating Saw Handpiece was developed as a power tool for cardiothoracic applications (Fig 4). It was thoroughly tested and approved by the Thoracic Expert Group.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO Foundation”
The brands and labels “approved by AO Technical Commission” and “approved by AO Foundation”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.