CONDUIT™ Lumbar Cages─Part of the CONDUIT™ Interbody Platform
Maarten Spruit, Matthew Scott-Young
The CONDUIT™ Interbody Platform is a comprehensive 3D printed solution that facilitates the various approaches achieving LIF. The CONDUIT™ Implant has also been developed to achieve fusion in the cervical spine. The cages come in various heights, footprints, and lordotic angles to address individual anatomical needs and to facilitate vertebral fusion (Fig 1).
Lumbar interbody fusion (LIF) is an established treatment for degenerative spinal disorders. Lumbar interbody fusion involves the placement of an implant (cage, spacer, or structural graft) within the intervertebral space after discectomy and end plate preparation.
Currently, LIF is performed using six main approaches:
- Posterior lumbar interbody fusion (PLIF)
- Transforaminal lumbar interbody fusion (TLIF)
- Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF)
- Oblique lumbar interbody fusion/anterior to psoas (OLIF/ATP)
- Lateral lumbar interbody fusion (LLIF)
- Anterior lumbar interbody fusion (ALIF)
Patient expectations and increasing demands for shorter hospital stays and early return to work have resulted in more innovative surgical techniques to reduce iatrogenic injury and postoperative morbidity. The development of new techniques attempts to shorten surgical times and achieve faster recovery with reduced complications.
The platform contains 3D printed cellular titanium implants that feature porous macro-, micro-, and nanostructures designed to mimic cortical and cancellous bone to facilitate intervertebral fusion.
CONDUIT™ Implants are designed with an interconnected pore structure making them 80% porous (700 μm pore size) compared with the typical 50–90% porosity of natural cancellous bone [1−6] (Fig 2).
In vitro studies [1] have reported more significant osteoblastic differentiation in human stem cells cultured on similar porous titanium constructs than solid titanium surfaces. In vivo studies with similar porous titanium materials show that bony in-growth increases at the 500−700 μm pore size range compared with larger or smaller pores [2−4].
All CONDUIT Implants undergo acid etching and heat treatment to promote microscale and nanoscale surface roughness. Surface roughness has been shown to benefit cell differentiation and proliferation in in vitro studies of osteoblast-like cells cultured on similar roughened titanium materials [7, 8]. Similar titanium materials with nanoscale features have been shown in in vitro studies [9] to increase osteoblast adhesion compared with conventional titanium materials.
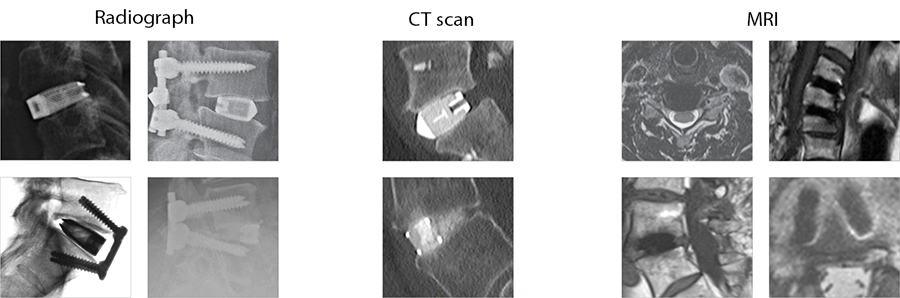
The implants' porous structure enables excellent visualization both intraoperatively and postoperatively on imaging modalities without interference (Fig 3).
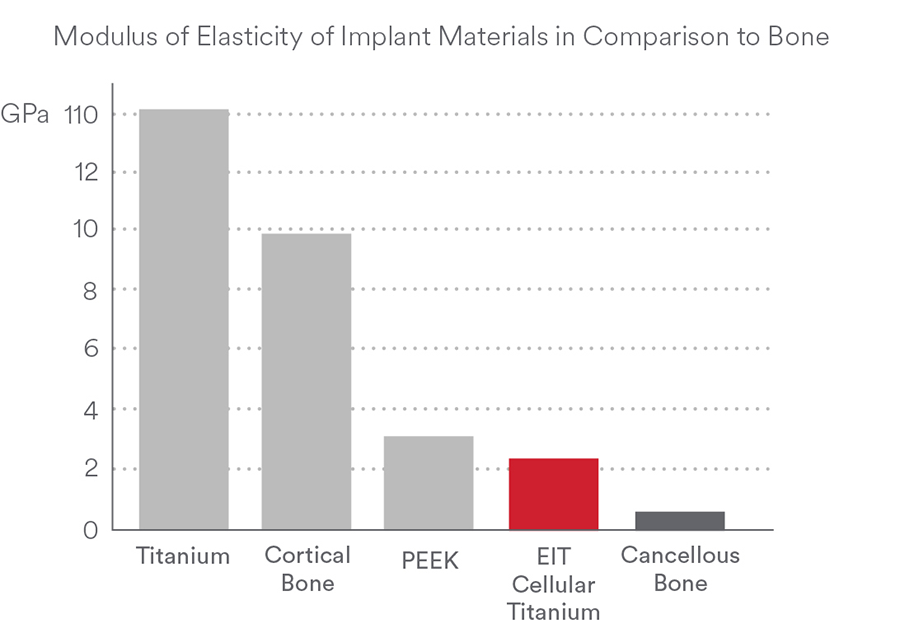
Proprietary 3D printing technology leads to a cellular design that offers a Modulus of Elasticity like bone (Fig 4) [10] aiming to reduce stress shielding and implant subsidence [11].
Conclusion
The CONDUIT™ Interbody Cage Platform provides a range of cages that have 3D printed Titanium Technology, attractive bone contact characteristics, and surface roughness to promote and to improve bony fusion. Nanoscale surface technology represents a significant innovation in intervertebral fusion science.
References
- Cheng A, Cohen D, Boyan B, et al. Laser-sintered constructs with bio-inspired porosity and surface micro/nano-roughness enhance mesenchymal stem cell differentiation and matrix mineralization in vitro. Calcif Tissue Int. 2016 Dec;99(6):625–637.
- Wu S-H, Li Y, Zang Y-Q, et al. Porous titanium-6 aluminum-4 vanadium cage has better osseointegration and less micromotion than a poly-ether-ether-ketone cage in sheep vertebral fusion. Artif Organs. 2013 Dec;37(12):E191−201.
- Taniguchi N, Fujibayashi S, Takemoto M, et al. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: an in vivo experiment. Mater Sci Eng C Mater Biol Appl. 2016 Feb;59:90−701.
- Fukuda A, Takemoto M, Saito T, et al. Osteoinduction of porous Ti implants with a channel structure fabricated by Selective Laser Melting. Acta Biomater. 2011 May; 7(5):2327−2336.
- Bostrom M, Boskey A, Kaufman J, et al. Form and function of bone. In: Orthopaedic Basic Science Biology and Mechanics of the Musculoskeletal System. 2nd ed. Rosemont, Illinois: American Academy of Orthopedic Surgeons; 2000:320−369.
- EIT Scaffold characteristics P773 Signed Report_DM 20180417.
- Olivares-Navarrete R, Hyzy SL, Gittens 1st RA, et al. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J. 2012 Nov;13(11):12:265−272.
- Lincks J, Boyan BD, Blanchard CR, et al. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998 Dec;19(23):2219−2232.
- Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004 Aug;25(19):4731−4739.
- Youngs Modulus comparison of various materials GUM00001 rev A/ VAL 2017-007.
- Wolff J. The Law of Bone Remodeling. Berlin Heidelberg New York: Springer; 1986 (translation of the German 1892 edition).
Case 1
(kindly provided by Matthew Scott-Young, MD, Gold Coast Spine, Gold Coast, Australia)
Patient medical history
A 72-year-old man with a 20-year history of chronic low back pain and radiculopathy to the right leg that was worse than to the left. An electromyography revealed bilateral L4, L5, and S1 radiculopathies. Conservative treatment failed. Discography triggered concordant pain in discs L3-4, L4-5, and L5-S1. Visual analog scale (VAS) backpain score was 90; VAS right leg pain score, 75; VAS left leg pain score, 65; Oswestry Disability Index, 56; and Roland-Morris Disability Questionnaire, 22.
Surgery
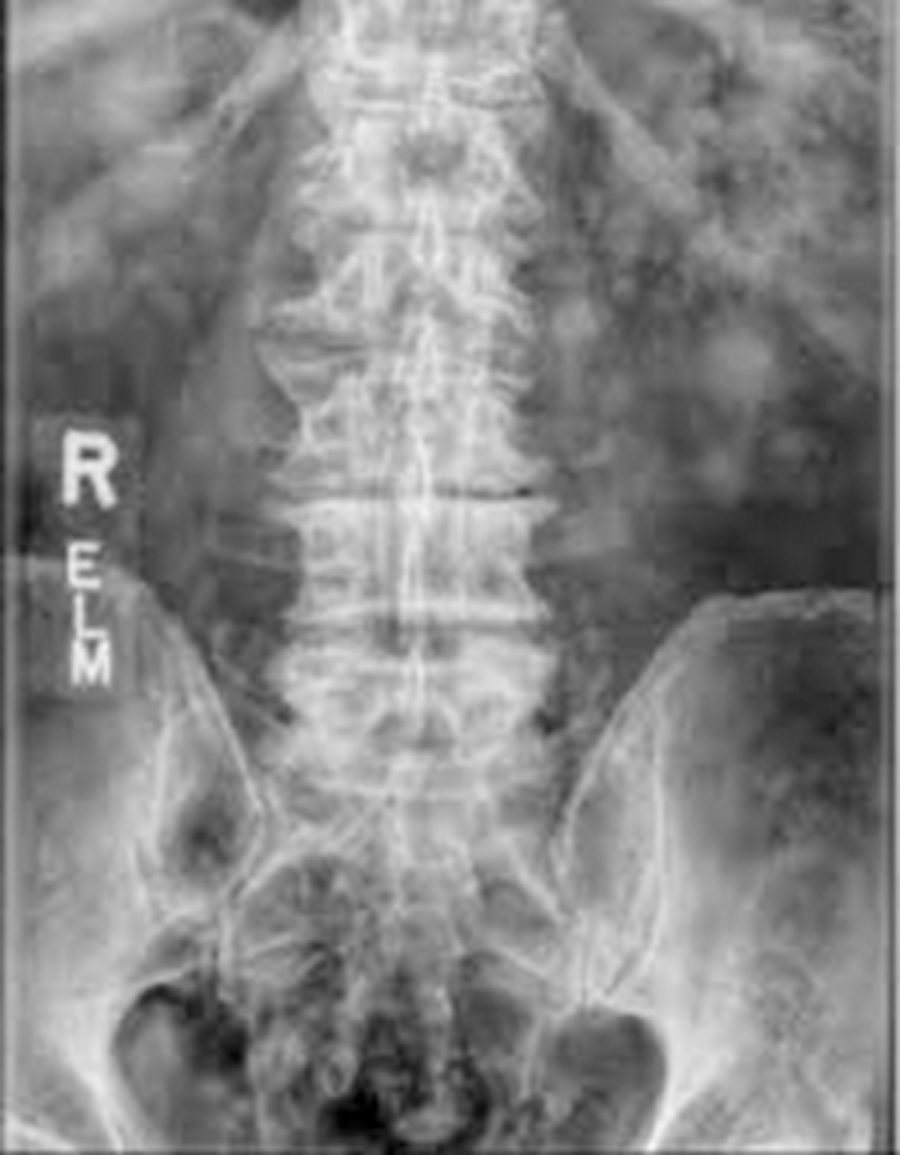
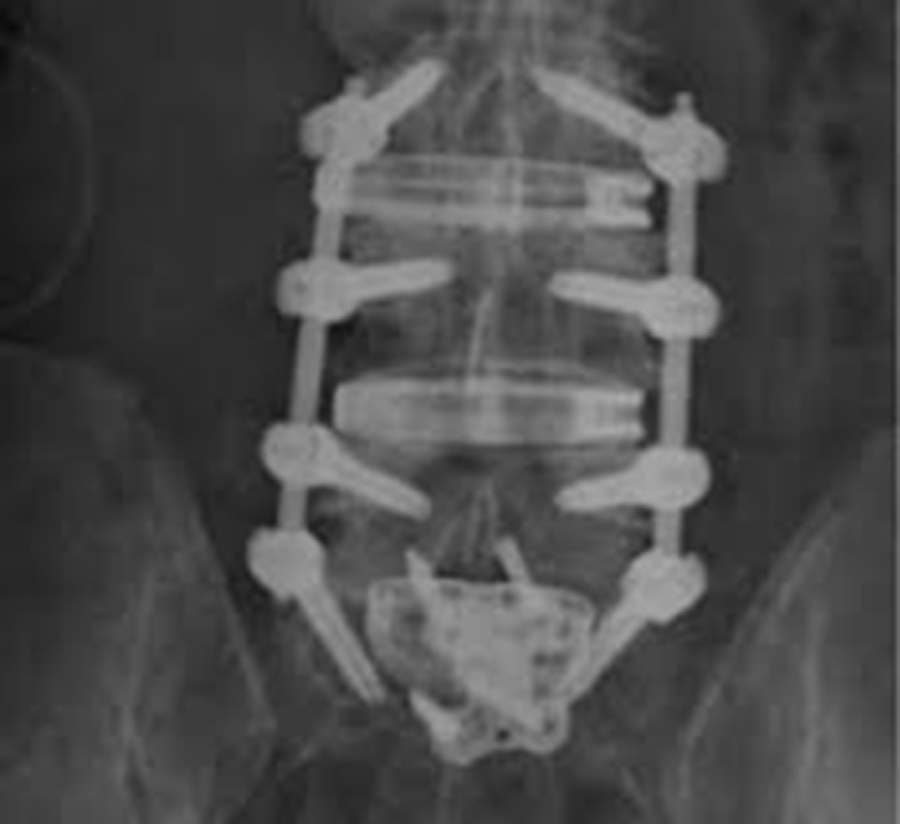
The patient underwent L3-S1 anterior reconstruction with indirect decompression (utilizing the UNLEASH™ ATP Procedural Solution L3-5 and ALIF L5-S1) and Percutaneous Posterior Segmental Pedicle Screw Fixation (Fig 5). Systems used were the following:
- INSIGHT® Lateral Access System (L3-L5)
- CONDUIT™ Lateral Interbody (L3-L5)
- CONDUIT™ ALIF Interbody and Aegis plate (L5-S1 with SYNFRAME® Access and Retractor System)
- VIPER PRIME® Screws: Posterior Pedicle Screw Instrumentation L3-S1
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO Foundation”
The brands and labels “approved by AO Technical Commission” and “approved by AO Foundation”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.