Cannulated Screw System CSS+
Michael Swords, Tim Schepers, Matthew Tomlinson, Andrew Sands
Cannulated screws are commonly used by orthopedic surgeons for the fixation of multiple fracture patterns and bone reconstructions. The screw cannulation allows placement over a guidewire to facilitate better accuracy before drilling or screw insertion. The use of cannulated screws adopts the principles of interfragmentary screw fixation and aims to optimize screw position.
In December 2021 the AO Trauma Technical Commission approved the Next Generation Cannulated Screw System Plus (CSS+) which was designed and developed by the Foot and Ankle Expert Group in collaboration with the AO primary industrial partner, DePuy Synthes.
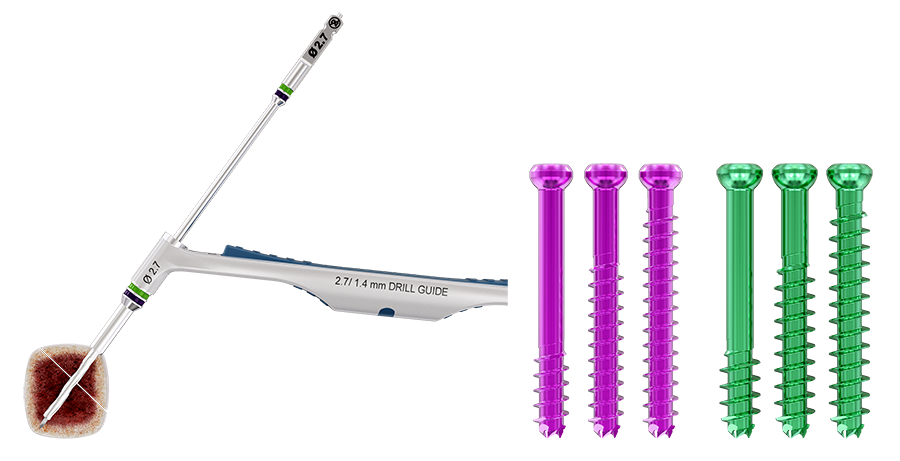
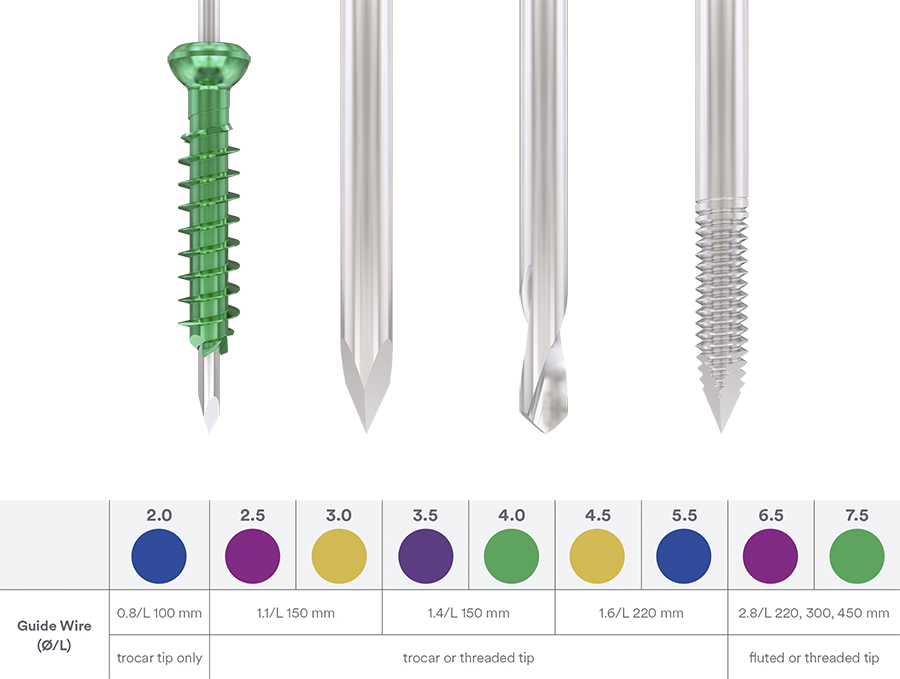
Ranging in diameter from 2.0–7.5 mm (with 0.5 mm diameter increments from 2.0– 4.5, and 1.0 mm increments from 5.5–7.5), the color-coded screws are available in titanium with short and long partial thread as well as fully threaded (Fig 1). All implants are available in sterile and non-sterile packaging. Color-coded washers are available for every screw size (Fig 2).
The Next Generation Cannulated Screw CSS+ is indicated for use in bone reconstruction, osteotomy, arthrodesis, joint fusion, fracture repair, and fracture fixation of bones appropriate for the size of the device. Color-coded instrumentation corresponding to its color-coded screw promotes ease of use and implantation (Fig 3).
In response to a clinical need for improved cutting performance the new and patented cannulated screw tip reduces the axial load needed to advance the screw; thereby achieving a more precise insertion with less, or no need for, pre-drilling and tapping (Fig 4).
The common problem of guidewire deflection was addressed with new cobalt chrome (CoCr) guidewires which provide a 29% higher bending stiffness compared with their stainless-steel counterparts. This ensures a straighter path and maintenance of the intended screw trajectory on insertion (Fig 5).
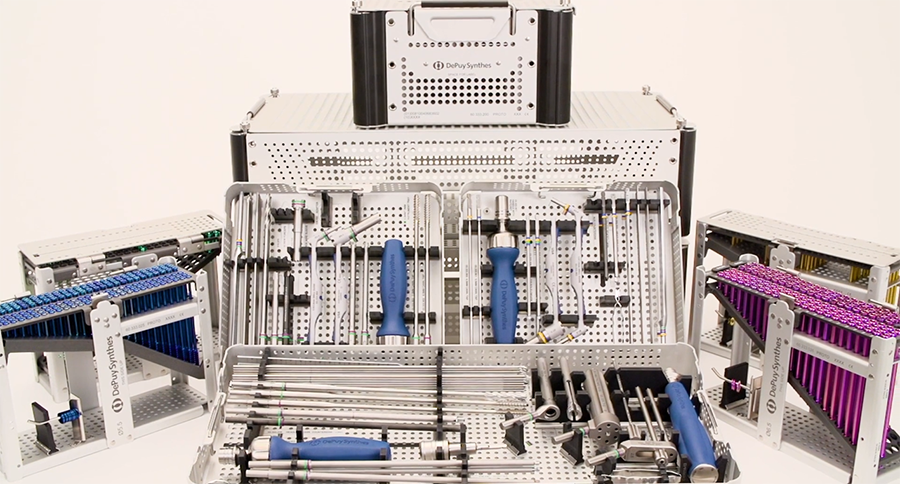
The system is neatly divided into preconfigured sets—small, medium, and large. These sets house separate screw racks and instrument trays according to size that is designed to provide efficiency in the operating room supply sourcing (Fig 6):
-
Small set: 2.0, 2.5, 3.0, 3.5, and 4.0 mm screws
-
Medium set: 4.5 and 5.5 mm screws
-
Large set: 6.5 and 7.5 mm screws
-
Large trauma set: 6.5 and 7.5 mm screws and long instruments specific for trauma use
Basic instruments in the sets are size appropriate and include guidewires, drill bits, drill guides, countersinks, measuring devices, screwdrivers with handles, and drive adaptor for power insertion. Additional instrumentation is provided for the medium set and large set trays which includes tissue protectors, guidewire sleeves, long drill bits, countersinks, screwdrivers, and guidewires. Three ergonomic screw handles are included in the set. Unique to the large trauma set (6.5 and 7.5 mm cannulated screws) is the inclusion of multiple and adjustable wire guides for accurate guide wire placement (Fig 7).
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO Foundation”
The brands and labels “approved by AO Technical Commission” and “approved by AO Foundation”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.